MIDTERM MODULE REPORT SPRING 2016 ANNA TRAYWICK INSTRUCTIONS: DO NOT DELETE THE QUESTIONS OR POINTS VALUES! (-2 pts if deleted) Complete the...
| Midterm Module report spring 2016 | |
INSTRUCTIONS:
DO NOT DELETE THE QUESTIONS OR POINTS VALUES! (-2 pts if deleted)
Complete the following INDIVIDUAL report. Work was done as a group, and as such it is acceptable if data tables and graphs look similar between group members. Answers requiring commentary, explanations, descriptions, etc., on the other hand, should sound unique to each individual. Plagiarism will be considered cheating and will be dealt with in accordance with the UAB Academic Honor Code.
All answers should be integrated into this Word document, including all graphs, charts, etc. Separate documents will not be opened and/or graded. All assignments should be turned in via Canvas As per the syllabus, reports submitted via email will not be graded.
Please use superscripts and subscripts where appropriate. A keyboard shortcut to subscripts: highlight the character then type CTRL-+. For superscripts: CTRL-Shift-+.
You answers should be thorough, and not only describe what happened but include how or why something happened.
Do not personalize procedures. Use complete sentences and watch your grammar.
Be sure to report standard deviation in the proper manner (see your manual).
Section 1: Data Collection, Reporting, and Results
1. Rates of Chemical Reactions Lab
a. (2 points) Using the initial rates method, report the order of the reaction with respect to iodate as determined from your data table. Use data from your notebook to show how you determined this value.
1 point: order; 1 point: data to support order
| Trial | | Time (s) | Rate (M/s) |
| 11.90 s | |||
| 7.83 s | |||
| 1.75 s | |||
| 1.50 s |
2nd Order
b. (2 points) Report the order of the reaction with respect to iodate that you determined graphically. Include the graph (prepared in a program like Excel) you prepared which allowed you to determine this value (axes labels, the trendline, and the linear regression must be present for any credit!!).
1 point: graph; 1 point: order of reaction
2nd Order
c. (3 points) Report your value for kobs. Compare the two methods available to determine kobs: using data from the table and graphically. Which method provides a more accurate value? Which method provides more information on the precision of the value and? Be sure to clearly explain your answers.
1 point: for kobs; 1 points: discussion of accuracy; 1 point: discussion of precision
2. Crystal Violet Lab
a. (3 points) Report the Beer’s Law calibration curve that you used to determine the relationship between absorbance and concentration of your crystal violet dilutions (axes labels, the trendline, and the linear regression must be present for any credit!!). What was the extinction coefficient? How does the magnitude of your y-intercept relate to the acceptability of your plot?
1 point: graph; 1 point: extinction coefficient; 1 point: y-intercept discussion
b. (3 points) Include the 3 integrated rate law plots that were used to determine the order with respect to the crystal violet (Use three graphs corresponding to either 0.1M or 0.2M NaOH) (axes labels, the trendline, and the linear regression must be present for any credit!!).
1 point each: 3 graphs
c. (3 points) Report the order, the rate constant, and the overall rate law expression for this reaction.
i. (1 point) The order of the reaction:
ii. (1 point) The value of the rate constant (with units):
iii. (1 point) The overall rate law expression:
3. Chemical Equilibrium and LeChatelier’s Principle Lab
a. (4 points) Explain clearly why the addition of water changes the equilibrium position, even though [H2O] does not appear in the equilibrium constant expression. Your explanation should include examples from your data, including a balanced equation (see your manual!), any color changes, and the dominant species in solution before and after the addition of H2O.
2 points: explanation; 1 point: equation; 1 point: species and observations
Balanced Equation:
Equation w/ Addition of Water:
b. (3 points) Use your observations from Exercise 3 to explain why it is important to include the temperature when reporting the value of K. Summarize the observations that you made as you changed the temperature of the cobalt solutions. Approximate the value of K (i.e., was it large or small) at 0 degrees C, room temperature, and 100 degrees C.
1 point: explanation; 1 point: observations; 1 point: approx. values of K
4. Acid and Base Solutions Lab
a. (4 points) Prepare graphs depicting the change in pH versus concentration for both a weak and strong acid using the data you collected in lab. Compare and contrast the behavior of aqueous solutions of these acids based on the shape of the curves. Include balanced equations in your comparison.
1 point each: 2 graphs; 1 point: comparison; 1 point: reactions
b. (4 points) In your own words, give a brief description of a buffer (what they are and how they work). What happens to the pH when a small quantity of a strong acid or base is added to a buffer solution? What happens to the pH of the buffer solution when a large quantity of acid or base is added? Explain your observations. Why is a buffer formed when HAc is added to NaOH?
2 points: description; 1 point: explanation of addition of acid/base; 1 point: buffer reasoning
A buffer is a solution that is resistant to pH changes and involves acids/bases along with the respective conjugates. Buffers are able to resist pH changes solely due to the conjugate acids and conjugate bases that are present in the solution(s). Adding a small quantity of a strong acid or strong base will not affect the pH of a buffer solution. This is due to the conjugate acid and conjugate base neutralizing the strong acid or strong base. When large quantities of an acid or base are added to a buffer, the system becomes overloaded and exceeds the buffer capacity. Therefore, the pH will greatly shift to very acidic or very basic depending on whether more strong acid or strong base is used in the solution. For HAc and NaOH, it becomes a buffer due to HAc being a weak acid and NaOH being a strong base. The HAc and NaOH neutralize each other to form a buffer solution.
Equation:
5. Determination of Ka from pH Lab
a. (3 points) In a short paragraph, describe the importance of taking small pH measurements around the equivalence and half-equivalence points. Include how you were able to estimate these points prior to collecting data. If we only use the pH at the half-equivalence point to calculate Ka, why is it still necessary to take small pH measurements around the equivalence point?
1 point each: equiv. and half-equiv. point discussion; 1 point: estimation of points
b. (2 points) Report the titration curve for one of your unknown acid trials (axes labels, the trendline, and title must be present for any credit!!). What was your endpoint volume? Approximate an equivalence point volume from your plot. How do these two values compare?
1 point: graph; 1 point: endpoint versus equivalence point discussion
c. (3 points) Report the Ka values of the unknown acid obtained by your group. How precise was your data? Describe some steps you can take to ensure high precision in titration results. Based on the precision, would you feel confident reporting these results to a scholarly journal? Why or why not?
point: values for Ka; 1 point: precision discussion; 1 points: journal discussion
Section 2: Application Problems
6a. (2 points) Using your textbook or the internet, lookup and report the pKa value of benzoic acid. Provide a reference for where you found this value.
pKa of benzoic acid = 4.19
Reference: http://sasc-specialists.ucdavis.edu/jim/118C/pKa's_of_various_Benzoic-Acetic_Acids.pdf
6b. (3 points) Clearly and concisely describe the difference in behavior of a strong and a weak acid and how the Ka of an acid indicates how an acid will behave. Provide details on how benzoic acid would behave based on its Ka.
6c. (6 points) Describe the protonation state of benzoic acid when it is in a solution of pH 3, pH 7, and pH 10. Is the protonation state of benzoic acid the same at any of these pHs? How can knowing this kind of information about your reactants help you with setting up successful reactions?
7a. (4 points) Some people store their batteries in the freezer to help them last longer. Using two different theories covered in lab up to this point, explain the chemistry behind this thought process.
7b. (3 points) Do you think that this can work to help the life of all batteries? Provide a justification for your answer.
7c. (3 points) Provide an argument as to whether or not there is a limit to the number of times this process can be repeated before it is ineffective.
8. (15 points total) After reading about the water crisis in Flint, Michigan, Polly wants to determine if there are any lead impurities in a water sample from her home faucet. She collects a sample and brings it to the lab to test. She wants to use her lab’s brand new isothermal titration calorimetry (ITC) machine so she reads the following information about how ITC works:
https://antisensescienceblog.wordpress.com/2013/11/15/experimental-techniques-explained-isothermal-titration-calorimetry-itc/
Polly decided that she would titrate her water sample with EDTA because she knows that EDTA can chelate (fancy word for form compounds with) lead with a 1 to 1 ratio. When she ran her experiment she obtained the following curve. From this, she concluded that there was indeed lead contamination in her water. Given this information, answer the questions below:
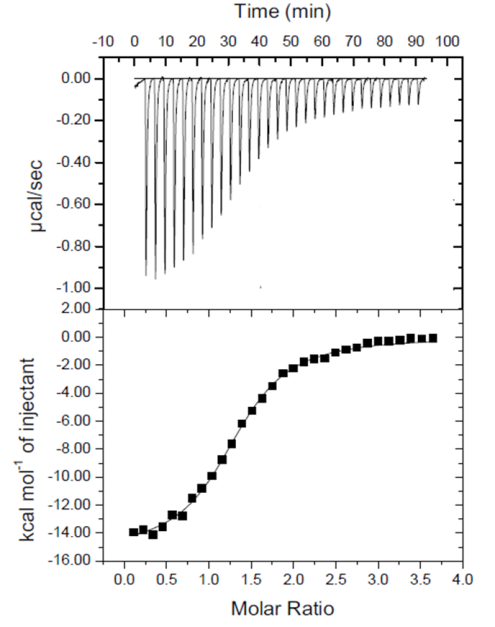

(2 points) Provide a balanced equation that shows how EDTA and lead form a chelate. (Your hints to how to do this are written in the problem above).
(2 points) Do you think that Polly titrated enough EDTA to chelate all metal ions in her water sample? Justify your answer by referring to the image above.
(3 points) List the axes used on the bottom graph in the image above. How do you think the shape of the graph helps you determine the stoichiometry of the reaction?
(2 points) What was the stoichiometry that Polly received from her ITC experiment? Give a reason why this value can differ from her expected stoichiometry.
(3 point) Considering the affinity that EDTA has for metal ions as shown in the table below, do you think that Polly was correct in concluding that she has a lead contamination in her water? Why or why not?
| Metal | Na | Li | Ba | Sr | Mg | Ca | Mn | Fe | Co | Zn | Cd | Pb | Ni |
| K (log) | 1.7 | 2.8 | 7.8 | 8.6 | 8.7 | 10.6 | 13.4 | 14.4 | 16.1 | 16.1 | 16.4 | 18.3 | 18.4 |
This Table shows EDTA-metal complex stability constants. The bigger the number, the tighter the EDTA grabs hold of the metal.
(3 points) What metal from the table above do you think Polly was chelating to EDTA? Give an explanation (include an example calculation…hint hint….).
BONUS (2 points): Provide reasonable justification as to whether or not Polly desires a cracker. Your answer should be concise but clear. Include references where necessary.



