To successfully complete this assignment, first read the following exercise from the Laboratory Manual: Exercise 41A Urinalysis. Reference: Martini, F., &Nath, J. (2009). Fundamentals of anatomy &
Blood composition depends on three major factors: diet, cellular metabolism, and urinary output. In 24 hours, the kidneys’ 2 million nephrons filter 150 to 180 liters of blood plasma through their glomeruli into the tubules, where it is selectively processed by tubular reabsorption and secretion. In the same period, urinary output, which contains by-products of metabolism and excess ions, is 1.0 to 1.8 liters. In healthy people, the kidneys can maintain blood constancy despite wide variations in diet and metabolic activity. Characteristics of Urine
Color and Transparency
Freshly voided urine is generally clear and pale yellow to amber in color. This normal yellow color is due to urochrome, a pigment metabolite that arises from the body’s destruction of hemoglobin and travels to the kidney as bilirubin or bile pigments. As a rule, color variations from pale yellow to deeper amber indicate the relative concentration of solutes to water in the urine. The greater the solute concentration, the deeper the color. Abnormal urine color may be due to certain foods, such as beets, various drugs, bile, or blood. Cloudy urine may indicate a urinary tract infection.
Odor
The odor of freshly voided urine is slightly aromatic, but bacterial action gives it an ammonia-like odor when left standing. Some drugs, vegetables (such as asparagus), and various disease processes (such as diabetes mellitus) alter the characteristic odor of urine. For example, the urine of a person with uncontrolled diabetes mellitus (and elevated levels of ketones) smells fruity or acetone-like.
pH
The pH of urine ranges from 4.5 to 8.0, but its average value, 6.0, is slightly acidic. Diet may markedly influence the pH of the urine. For example, a diet high in protein (meat, eggs, cheese) and whole wheat products increases the acidity of urine. Conversely, a vegetarian diet usually increases the alkalinity of the urine. A bacterial infection of the urinary tract may also cause the urine to become more alkaline.
Specific Gravity
Specific gravity is the relative weight of a specific volume of liquid compared with an equal volume of distilled water. The specific gravity of distilled water is 1.000, because 1 ml weighs 1 g. Since urine contains dissolved solutes, it weighs more than water, and its customary specific gravity ranges from 1.001 to 1.030. Urine with a specific gravity of 1.001 contains few solutes and is considered very dilute. Dilute urine commonly results when a person drinks excessive amounts of water, uses diuretics, or suffers from diabetes insipidus or chronic renal failure. Conditions that produce urine with a high specific gravity include limited fluid intake, fever, diabetes mellitus, gonorrhea, and kidney inflammation, called pyelonephritis. If urine becomes excessively concentrated, some of the substances normally held in solution begin to precipitate or crystallize, forming kidney stones, or renal calculi.
Water is the largest component of urine, accounting for 95% of its volume. The second largest component of urine is urea. Nitrogenous wastes in the urine include urea, uric acid, and creatinine. Urea comes from the breakdown of proteins. Uric acid is a breakdown product from nucleic acids. Creatinine is a metabolite produced from the metabolism of creatine phosphate in muscle tissue.
Normal solute constituents of urine, in order of decreasing concentration, include urea, sodium, potassium, phosphate, and sulfate ions; creatinine; and uric acid. Much smaller but highly variable amounts of calcium, magnesium, and bicarbonate ions are also found in the urine. Abnormally high concentrations of any of these urinary constituents may indicate a pathological condition. Abnormal Urinary Constituents
Abnormal urinary constituents are substances not normally present in the urine when the body is operating properly.
When certain pathological conditions are present, urine composition often changes dramatically. Table 41.1 identifies substances that are not normally found in the urine and describes their characteristics.
Casts
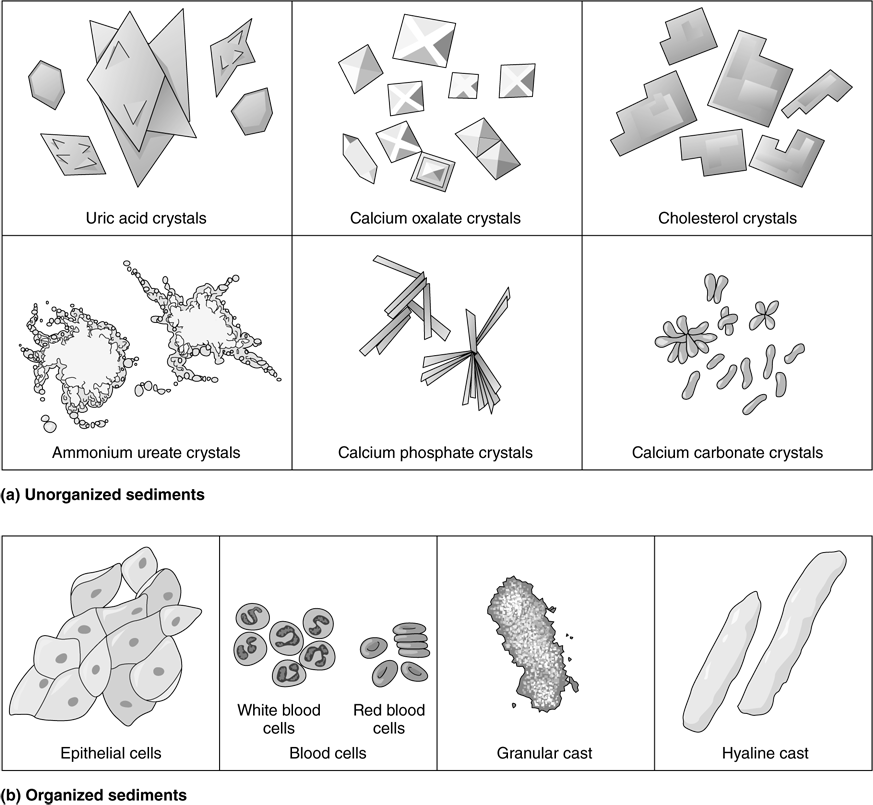
Any complete discussion of the varieties and implications of casts is beyond the scope of this exercise. However, because they always represent a pathological condition of the kidney or urinary tract, they should at least be mentioned. Casts are hardened cell fragments, usually cylindrical, which are formed in the distal convoluted tubules and collecting ducts and then flushed out of the urinary tract. Hyaline casts are formed from a mucoprotein secreted by tubule cells (Figure 41.1b, p. 632). These casts form when the filtrate flow rate is slow, the pH is low, or the salt concentration is high, all conditions that cause protein to denature. Red blood cell casts are typical in glomerulonephritis, as red blood cells leak through the filtration membrane and stick together in the tubules. White blood cell casts form when the kidney is inflamed, which is typically a result of pyelonephritis (a type of urinary tract infection) but sometimes occurs with glomerulonephritis. Degenerated renal tubule cells form granular casts (Figure 41.1b).
Activity 1 Analyzing Urine Samples
In this part of the exercise, you will use prepared dipsticks and perform chemical tests to determine the characteristics of normal urine as well as to identify abnormal urinary components. You will investigate two or more urine samples. The first, designated as the standard urine specimen in the Activity 1 chart (p. 631), will be either yours or a “standard” sample provided by your instructor. The second will be an unknown urine specimen provided by your instructor. Make the following determinations on both samples, and record your results by circling the appropriate item or description or by adding data to complete the chart. If you have more than one unknown sample, accurately identify each sample by number.
Table 41.1 Abnormal Urinary Constituents
Abnormal urinary constituent Clinical term Description Possible conditions
Glucose Glycosuria (glucosuria) High blood sugar levels due to inadequate insulin levels; or can result when active transport mechanisms for glucose are exceeded temporarily
Pathological: uncontrolled diabetes mellitus
Nonpathological: Excessive carbohydrate intake
Protein Proteinuria (albuminuria) Increased permeability of the glomerular filtration membrane (proteins are usually too large to pass through); albumin is the most abundant blood protein
Pathological: hypertension, glomerulonephritis, ingestion of poisons, bacterial toxins, kidney trauma
Nonpathological: excessive physical exertion, pregnancy
Ketone bodies Ketonuria Excessive production of intermediates of fat metabolism, which may result in acidosis Uncontrolled diabetes mellitus, starvation, low-carbohydrate diets
Erythrocytes (RBCs) Hematuria Irritation of the urinary tract organs that results in bleeding; or a result of leakage of RBCs through a damaged filtration membrane
Bleeding in the tract: kidney stones, urinary tract tumors, trauma to urinary tract organs
Damaged filtration membrane: glomerulonephritis
Hemoglobin Hemoglobinuria Fragmentation of erythrocytes, resulting in the release of hemoglobin into the plasma and subsequently into the filtrate Hemolytic anemia, transfusion reactions, severe burns, poisonous snake bites, renal disease
Nitrites Nitrituria Results when gram-negative bacteria such as E. coli reduce nitrates to form nitrites Urinary tract infections (UTIs)
Bile pigments Bilirubinuria Increased levels of bilirubin in the urine as a result of liver damage or blockage of the bile duct Hepatitis, cirrhosis of the liver, gallstones
Leukocytes (WBCs) Pyuria Presence of WBCs or pus in the urine caused by inflammation of the urinary tract Urinary tract infections (including pyelonephritis), gonorrhea
Obtain and wear disposable gloves throughout this laboratory session. Although the instructor- provided urine samples are actually artificial urine (concocted in the laboratory to resemble real urine), you should still observe the techniques of safe handling of body fluids as part of your learning process. When you have completed the laboratory procedures: (1) dispose of the gloves, used pH paper strips, and dipsticks in the autoclave bag; (2) put used glassware in the bleach-containing laboratory bucket; (3) wash the lab bench down with 10% bleach solution.
Determination of the Physical Characteristics of Urine
Determine the color, transparency, and odor of your “standard” sample and one of the numbered pathological samples, and circle the appropriate descriptions in the Activity 1 chart.
Obtain a roll of wide-range pH paper to determine the pH of each sample. Use a fresh piece of paper for each test, and dip the strip into the urine to be tested two or three times before comparing the color obtained with the chart on the dispenser. Record your results in the chart. (If you will be using one of the combination dipsticks—Chemstrip or Multistix—you can use these dipsticks to determine pH.)
To determine specific gravity, obtain a urinometer cylinder and float. Mix the urine well, and fill the urinometer cylinder about two-thirds full with urine.
Examine the urinometer float to determine how to read its markings. In most cases, the scale has numbered lines separated by a series of unnumbered lines. The numbered lines give the reading for the first two decimal places. You must determine the third decimal place by reading the lower edge of the meniscus—the curved surface representing the urine-air junction—on the stem of the float.
Carefully lower the urinometer float into the urine. Make sure it is floating freely before attempting to take the reading. Record the specific gravity of both samples in the chart. Do not dispose of this urine if the samples that you have are less than 200 ml in volume because you will need to make several more determinations.
Determination of Inorganic Constituents in Urine
Sulfates
Using a 10-cc graduated cylinder, add 5 ml of urine to a test tube, and then add a few drops of dilute hydrochloric acid and 2 ml of 10% barium chloride solution. The appearance of a white precipitate (barium sulfate) indicates the presence of sulfates in the sample. Clean the graduated cylinder and the test tubes well after use. Record your results.
Phosphates
Obtain a hot plate and a 500-ml beaker. To prepare the hot water bath, half fill the beaker with tap water and heat it on the hot plate. Add 5 ml of urine to a test tube, and then add three or four drops of dilute nitric acid and 3 ml of ammonium molybdate. Mix well with a glass stirring rod, and then heat gently in a hot water bath. Formation of a yellow precipitate indicates the presence of phosphates in the sample. Record your results.
Chlorides
Place 5 ml of urine in a test tube, and add several drops of silver nitrate. The appearance of a white precipitate (silver chloride) is a positive test for chlorides. Record your results.
Nitrites
Use a combination dipstick to test for nitrites. Record your results.
Determination of Organic Constituents in Urine
Individual dipsticks or combination dipsticks (Chemstrip or Multistix) may be used for many of the tests in this section. If you are using combination dipsticks, be prepared to take the readings on several factors (pH, protein [albumin], glucose, ketones, blood/hemoglobin, leukocytes, urobilinogen, bilirubin, and nitrites) at the same time. Generally speaking, results for all of these tests may be read during the second minute after immersion, but readings taken after 2 minutes have passed should be considered invalid. Pay careful attention to the directions for method and time of immersion and disposal of excess urine from the strip, regardless of the dipstick used. Identify the dipsticks that you use in the chart. If you are testing your own urine and get an unanticipated result, it is helpful to know that most of the combination dipsticks produce false positive or negative results for certain solutes when the subject is taking vitamin C, aspirin, or certain drugs.
Urea
Put two drops of urine on a clean microscope slide and carefully add one drop of concentrated nitric acid to the urine. Slowly warm the mixture on a hot plate until it begins to dry at the edges, but do not allow it to boil or to evaporate to dryness. When the slide has cooled, examine the edges of the preparation under low power to identify the rhombic or hexagonal crystals of urea nitrate, which form when urea and nitric acid react chemically. Keep the light low for best contrast. Record your results.
Glucose
Use a combination dipstick or obtain a vial of Clinistix, and conduct the dipstick test according to the instructions on the vial. Record your results in the Activity 1 chart.
Because the Clinitest reagent is routinely used in clinical agencies for glucose determinations in pediatric patients, it is worthwhile to conduct this test as well. Obtain the Clinitest tablets and the associated color chart. You will need a timer (watch or clock with a second hand) for this test. Using a medicine dropper, put 5 drops of urine into a test tube; then rinse the dropper and add 10 drops of water to the tube. Add a Clinitest tablet. Wait 15 seconds and then compare the color obtained to the color chart. Record your results.
Protein
Use a combination dipstick or obtain the Albustix dipsticks, and conduct the determinations as indicated on the vial. Record your results.
Ketones
Use a combination dipstick or obtain the Ketostix dipsticks. Conduct the determinations as indicated on the vial. Record your results.
Blood/Hemoglobin
Test your urine samples for the presence of hemoglobin by using a Hemastix dipstick or a combination dipstick according to the directions on the vial. Usually a short drying period is required before making the reading, so read the directions carefully. Record your results.
Bilirubin
Using a combination dipstick, determine if there is any bilirubin in your urine samples. Record your results.
Also conduct the Ictotest for the presence of bilirubin. Using a medicine dropper, place one drop of urine in the center of one of the special test mats provided with the Ictotest reagent tablets. Place one of the reagent tablets over the drop of urine, and then add two drops of water directly to the tablet. If the mixture turns purple when you add water, bilirubin is present. Record your results.
Leukocytes
Use a combination dipstick to test for leukocytes. Record your results.
Urobilinogen
Use a combination dipstick to test for urobilinogen. Record your results.
Clean up your area following the procedures described at the beginning of this activity.
Activity 1: Urinalysis Results
Observation or test Normal values Standard urine specimen Unknown specimen (# )
Physical Characteristics
Color Pale yellow Yellow: pale medium dark other Yellow: pale medium dark other
Transparency Clear Clear Slightly cloudy Cloudy Clear Slightly cloudy Cloudy
Odor Aromatic Describe: Describe:
pH 4.5–8.0
Specific gravity 1.001–1.030
Inorganic Components
Sulfates Present Present Absent Present Absent
Phosphates Present Present Absent Present Absent
Chlorides Present Present Absent Present Absent
Nitrites Absent Present Absent Present Absent
Dipstick:
Organic Components
Urea Present Present Absent Present Absent
Glucose Dipstick: Negative Record results: Record results:
Clinitest Negative
Protein Dipstick: Negative
Ketone bodies Dipstick: Negative
RBCs/hemoglobin Dipstick: Negative
Bilirubin Dipstick: Negative
Ictotest Negative (no color change) Negative Positive (purple) Negative Positive (purple)
Leukocytes Absent Present Absent Present Absent
Dipstick:
Urobilinogen Present Present Absent Present Absent
Dipstick:
Activity 2 Analyzing Urine Sediment Microscopically (Optional)
If your instructor so indicates, conduct a microscopic analysis of urine sediment in “real” urine. The urine sample to be analyzed microscopically has been centrifuged to spin the more dense urine components to the bottom of a tube, and some of the sediment has been mounted on a slide and stained with Sedi-stain to make the components more visible.
Go to the demonstration microscope to conduct this study. Using the lowest light source possible, examine the slide under low power to determine whether you can see any common sediments (Figure 41.1).
Unorganized sediments: Chemical substances that form crystals or precipitate from solution; for example, calcium oxalates, carbonates, and phosphates; uric acid; ammonium ureates; and cholesterol. Also, if one has been taking antibiotics or certain drugs such as sulfa drugs, these may be detectable in the urine in crystalline form. Normal urine contains very small amounts of crystals, but conditions such as urinary retention or urinary tract infection may cause the appearance of much larger amounts. The high-power lens may be needed to view the various crystals, which tend to be much more minute than the organized cellular sediments.
Organized sediments: Include epithelial cells (rarely of any pathological significance), white blood cells, red blood cells, and casts. The presence of white blood cells, red blood cells, and casts other than trace amounts always indicates kidney pathology. Note that red blood cells, white blood cells, and epithelial cells can also form casts.
Figure 41.1 Examples of sediments.