BIOLOGY LAB REPORT
EXPERIMENT
Microscopic Examination of Living
Microorganisms Using a Hanging-
Drop Preparation or a Wet Mount
Learning Objectives
Once you have completed this experiment, you should know how to
1. Microscopically examine living microorganisms.
2. Make a hanging-drop preparation or wet mount to view living microorganisms.
Principle
Bacteria, because of their small size and a refractive index that closely approximates that of water, do not lend themselves readily to microscopic examination in a living, unstained state. Examination of living microorganisms is useful, however, o do the following:
1. Observe cell activities such as motility and binary fission.
2. Observe the natural sizes and shapes of the cells, considering that heat fixation (the rapid passage of the smear over the Bunsen burner flame) and exposure to chemicals during staining cause some degree of distortion.
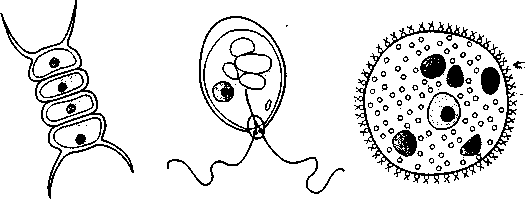
In this experiment you will use individual cultures of Pseudomonas aeruginosa, Bacillus cereus, Staphylococcus aureus, and Proteus vulgaris for a hanging-drop preparation or a wet mount. Hay infusion or pond water may be substituted or used in addition to the above organisms. Figure 5.1 illustrates several organisms commonly found in pond water and hay infusions.
Algae
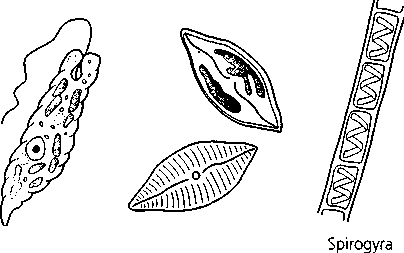
Euglena
Diatoms
Scenedesmus
Chlamydomonas
Volvox
Protozoa
Paramecium
Stylonychia
Amoeba
Heteronema
Vorticella
Figure 5.1 Algae and protozoa commonly found in natural infusions and pond water (drawings are not to scale)
You will observe the preparation(s) microscopically for differences in the sizes and shapes of the cells, as well as for motility, a self-directed movement. It is essential to differentiate between actual motility and Brownian movement, a vibratory movement of the cells due to their bombardment by water molecules in the suspension. Hanging-drop preparations and wet mounts make the movement of microorganisms easier to see because they slow down the movement of water molecules.
AT THE BENCH
Materials
Cultures
24-hour broth cultures of P. aeruginosa, B. cereus, S. aureus, and P. vulgaris; and/or hay infusion broth cultures or pond water. (See Appendix 3 for the preparation of hay infusion broth.)
Equipment
Bunsen burner, inoculating loop, depression slides, glass slides, coverslips, microscope, petroleum jelly, and cotton swabs.
Procedure
Hanging-Drop Preparation
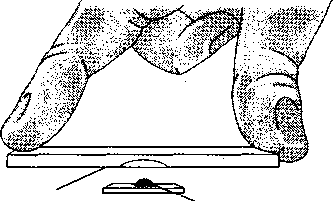
Perform the following steps for each culture provided in this experiment. Steps 1-4 are illustrated in Figure 5.2.
1. With a cotton swab, apply a ring of petroleum jelly around the concavity of the depression slide.
2. Using aseptic technique, place a loopful of the culture in the center of a clean coverslip.
3. Place the depression slide, with the concave surface facing down, over the coverslip so that the depression covers the drop of culture. Press the slide gently to form a seal between the slide and the coverslip.
4. Quickly turn the slide right side up so that the drop continues to adhere to the inner surface of the coverslip.
5. For microscopic examination, first focus on the drop culture under the low-power objective (10X) and reduce the light source by adjusting the Abbe condenser. Repeat using the high-power objective (40X).
6. Examine the hanging-drop preparation and record your observations in the Lab Report.
Wet Mount
A wet mount may be substituted for the hanging-drop preparation using a similar procedure:
1. With a cotton swab apply a thin layer of petroleum jelly along the edge of the four sides of a coverslip.
2. Using aseptic technique, place a loopful of the culture in the center of a clean coverslip.
3. Place a clean glass slide over the coverslip and press the slide gently to form a seal between the slide and the coverslip.
4. Follow Steps 4 and 5 in the hanging-drop procedure.
5. Examine the wet-mount preparation and record your observations in the Lab Report.
PROCEDURE
Loopful
Of bacterial
Culture -
Slide concavity
PDepression slide
Spread a ring of petroleum jelly around the concavity
of the depression slide.
Coverslip
Place a loopful of the bacterial culture in the center of the coverslip.
Culture drop
Petroleum jelly
Coverslip
Depression slide
Hanging-drop preparation
Coverslip ^Culture drop
Lower the depression slide, with the concavity facing down, onto the coverslip. Press gently to form a seal.
Figure 5.2 Hanging-drop preparation
Turn the hanging-drop preparation over so that the culture drop adheres to the coverslip.
Lab Report
Observations and Results
1. Examine the hanging-drop or wet-mount preparation to determine shape and motility of the different bacteria present. Record your results in the chart below.
| Organisms | Shape | True Motility or Brownian Movement? |
| S. aureus | | |
| P. aeruginosa | | |
| B. cereus | | |
| P. vulgaris | | |
2. Draw a representative field of each of the above organisms.
S. aureus P. aeruginosa
B. cereus P. vulgaris
3. Draw representative fields of pond water and hay infusion if you used them. Try to identify some of the organisms that you see by referring to Figure 5.1. Note the shape and type of movement in the chart below.
Pond water Hay infusion
| | Pond Water | Hay Infusion | ||||
| Shape | | | | | | |
| True motility or Brownian movement? | | | | | | |
| Organism | | | | | | |
Review Questions
Why are living, unstained bacterial preparations more difficult to observe than stained preparations?
What is the major advantage of using living cell preparations (hanging-drop or wet mount) rather than stained preparations?
3. How do you distinguish between true motility and Brownian movement?
4. During the microscopic observation of a drop of stagnant pond water, what criteria would you use to distinguish viable organisms from nonviable suspended debris?



