Paraphrasing paper
Introduction
Hydrogen sulfide (H2S) is a gas that naturally occurs in hot springs, crude petroleum, and even natural gas. This gas is usually produced by the breakdown of organic materials commonly found in wastes of human and animals by bacteria. Some of the activities that commonly result to the production of hydrogen sulfide gas include treatment of wastewater, drilling and even refining of petroleum or natural gas (Occupational Safety and Health Administration).
Aside from being in a form of gas, hydrogen sulfide may also exist as a gas, which is compressed to become a liquid. It is commonly found in places which are enclosed and have poor ventilation because it travels along the ground as a result of its heavier weight than air. Some of the common locations of hydrogen sulfide include basements, and sewer lines (Occupational Safety and Health Administration).
H2S is becoming public concern, especially because enormous number of industries use H2S in their manufacture. Hence, enormous number of workers are exposed to it. In this report I am going to thoroughly explain H2S properties, common use of H2S, health effects, and the government regulation of H2S in workplace. In addition, methods to control and limit H2S exposure, case study, and finally conclusion.
H2S Properties
Similar to other compounds, hydrogen sulfide (H2S) also has different properties and components. Based on its chemical family, hydrogen sulfide is a part of the group of inorganic gas or inorganic sulfide. It is physically colorless, however it can be seen as a liquid at extremely low temperatures or extremely high pressures. In terms of odor, this gas has a smell similarly to rotten eggs at very low concentrations. However, at 30-100 ppm, its odor becomes sweet. It has a molecular weight of 34.08, melting point of -85.5°C, and boiling point of -60.3°C (“CHEMINFO: Hydrogen Sulfide”).
When dealing with the solubility of the gas, it has been revealed that it is slightly soluble in water and has variety of solubility in other liquids. For example, ethanol, methanol, acetone, gasoline, kerosene, and other oils are substances where hydrogen sulfide is soluble in. Since the compound is a gas in form, its pH value cannot be computed. However, when it exists as a liquid, the pH for the compound is approximately 4.1. Lastly, the critical pressure and temperature of this compound is 9006-9008 kPa and 100.4°C respectively (“CHEMINFO: Hydrogen Sulfide”).
Hydrogen sulfide gas has a density of 2.07 g/cu cm and a vapor density of approximately 7.837 at 470 °C. It has a viscosity of 0.17 at 120°C, 0.008 at 140°C, 0.0064 at 158°C, and 5.952 at 160°C (Nehb and Vydra, 2006). In terms of iconicity, hydrogen sulfide is at 10.46 eV. On the other hand, for its spectral properties, the index of refraction is 1.947 for alpha and 2.038 for beta (Lewis, 2007).
Common Use
This compound is commonly used in the production of various metallic sulfides. Other industries use this compound for the production of other compounds such as phosphors and oil additives, which are substance added slightly to compounds usually to improve its quality. Moreover, hydrogen sulfide is used as to analyse other chemicals in analysis procedure. Further more, it’s used as by-product in multiple fields like petroleum production, mining, textile manufacture, food processing, and agricultural soils. Lastly, it is also used as remover of impurities in metals and for the reaction of other metals with various organic compounds (“CHEMINFO: Hydrogen Sulfide”).
Exposure and Health Effects
Since hydrogen sulfide is considered as a toxic and irritable gas, it is important that the presence of such gas is detected early to be able to protect the health of individuals and employees. The health effects of hydrogen sulfide can be classified based on the route by which it enters the body.
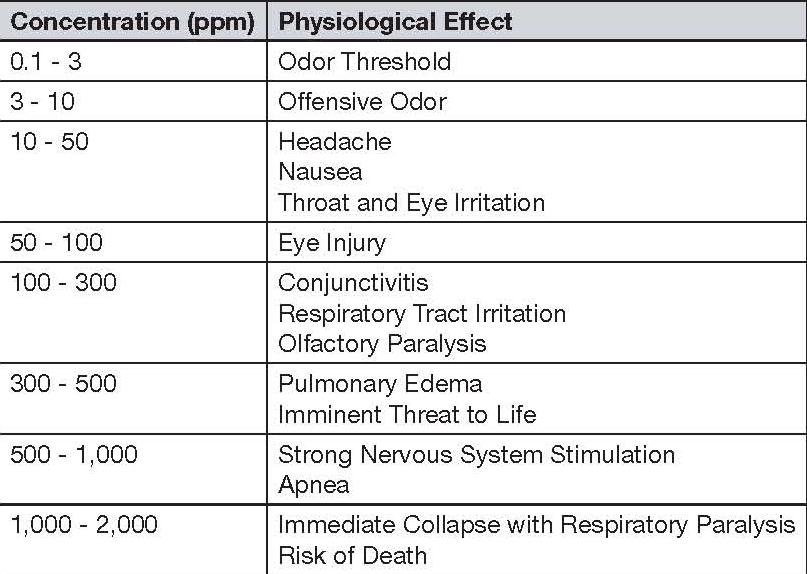
When an individual is exposed to hydrogen sulfide through inhalation, exposure to 0.01-1.5 ppm of hydrogen sulfide will already allow the person to smell the characteristic rotten egg smell of the gas. On the other hand, exposure to 2-5 ppm of hydrogen sulfide concentration can lead to nausea, watery eyes or teary eyes, apnea, and even headaches. In asthma patients, exposure to this concentration may already lead to airway problems such as bronchial constriction. Meanwhile, at 20 ppm concentration of hydrogen sulfide, an individual already experiences fatigue, increased irritability, loss of appetite, nausea, vertigo, and poor memory (“United States Department of Labor”).
Higher concentrations of hydrogen sulfide such as 50-100 ppm may lead to a state commonly known as gas eye. Moreover, continuous exposure to this chemical at 50-100 ppm after 1 hour can lead to the irritation of the respiratory system. Defects in the digestive system and the person’s loss of appetite are also some of the health effects associated with exposure to hydrogen sulfide levels of 50-100 ppm. At 100 ppm concentration of hydrogen sulfide gas, the individual exposed is at high risk of experiencing symptoms such as coughing, irritation of the eyes, loss of smell, drowsiness, irritation of the throat after an hour of exposure to this concentration, and even death when the length of exposure reaches 48 hours (“United States Department of Labor”).
On the other hand, when an individual is exposed to hydrogen sulfide through direct skin contact, freezing of the tissues commonly referred to as frostbite can occur. Some of the known symptoms of this phenomenon include prickling and itching along with numbness of the area in direct contact with the hydrogen sulfide. During severe exposure, the production of blisters can occur. However, in worse circumstances, the death of the tissues is also possible (“CHEMINFO: Hydrogen Sulfide”).
When compared to the other modes of transmission, the most commonly reported effect of exposure to hydrogen sulfide is the irritation of the eyes. It has been reported that inflammation and irritation of the eyes can actually occur even at very-low concentrations. Other symptoms of hydrogen sulfide exposure include light sensitivity, tearing of the eyes, burning sensation in the eyes, and even blurred visions (“CHEMINFO: Hydrogen Sulfide”).
Government Regulation
In terms of government regulation, the Occupational Safety & Health Administration (OSHA) proposed different requirements for the evaluation and control of exposures to hydrogen sulfide. For general industry, it is required to have proper ventilation and safety management of the chemicals, which are considered as highly hazardous. It is also expected that individuals who are at high risk of being exposed to hydrogen sulfide be provided with proper personal protective equipment especially for respiratory protection. There is also worker exposure limits proposed by the same organization. For a general industry, exposures to hazardous chemicals should not exceed 20 ppm. For construction, the exposure of individuals to gases, vapors, and even dusts and mists should be set at 10 ppm. Lastly, air contaminants should have an exposure limit of 10 ppm (“United States Department of Labor”).
Control Method
Some of the common control methods for hydrogen sulfide include provision of local exhaust ventilation along with the usage of proper respiratory protection devices. It is also possible for the hydrogen sulfide to be measured and recovered to reduce the exposure of individuals to high concentrations of the cases. The provision of water discharge, which is continuous to the sewage system and proper covering and venting of the drains of wastes, is another control method (“United States Department of Labor”).
Case Studies
There are also case studies focused on hydrogen sulfide exposure, in this case study, a total of 3 industrial incidents caused by exposure to hydrogen sulfide have been presented. In these cases, the use of biological monitoring was thoroughly discussed to provide evidences for the regulatory enforcement of hydrogen sulfide. For the incidents considered as non-fatal, urine samples are to be submitted at two or more time points. These time should be between the time of the incident and 15-h post exposure. Based on the results, hydrogen sulfide concentration of 12 ppm for 30 minutes can be detected using biological monitoring (Jones, pp. 374).
Conclusion
To sum up, hydrogen sulfide gas is a colorless, odorless, flammable, extremely hazardous gas that has a smell of rotten eggs. It can cause multiple health effects range from mild like nausea, headache, and loss of appetite to more sever effects like eye and skin irritation, and/or even death at extremely high concentrations. Therefore, different government agencies proposed some regulatory guidelines and regulations aiming to control and limit the exposure of hydrogen sulfide by workers in workplaces.
Recommendation
Based on the given information and the case study, hydrogen sulfide is capable of causing acute effects including eyes irritation, skin irritation, respiratory problems, among other problems and these incidents do occur in general industries, the concentration level should be reduced to 10ppm in workplaces in order to minimize health defects for workers.
Works Cited
“CHEMINFO: Hydrogen Sulfide.” CHEMINFO: Hydrogen Sulfide. Canadian Centre for Occupational Health & Safety, 2012. Web. 08 Apr. 2016
Jones, K. “Case studies of hydrogen sulphide occupational exposure incidents in the UK.” Toxicology Letters 231.3 (2014): 374-377. Web.
Lewis, R.J. Sr. Hawley’s Condensed Chemical Dictionary 15th edition. New York, New York: John Wiley & Sons, Inc., 2007. Print.
Nehb, W. and Vydra, K. Ullmann’s Encyclopedia of Industrial Chemistry. 7th ed. (1999-2011). New York, NY: John Wiley & Sons, 2006. Print.
Occupational Safety and Health Administration. “OSHA Fact Sheet: Hydrogen Sulfide.” OSHA. 2005. Print.
“United States Department of Labor.” Safety and Health Topics Web. 10 Apr 2016.



