Diagnosis advancement in haematological Neoplas ADVANCES IN THE LABORATORY DIAGNOSIS OF HAEMATOLOGICAL NEOPLASM
HAEMATOLOGICAL NEOPLASM 21
ADVANCES IN THE LABORATORY DIAGNOSIS OF HAEMATOLOGICAL NEOPLASM
Name of Student:
Course:
Name of Instructor:
Date
Abstract
The study aims at reviewing haematological neoplasm as a subject, its causes, symptoms, and prevention. It will also discuss the current advances in the laboratory diagnosis of haematological neoplasm. This is to help understand wider and better how this disease gets to the body and its ability while in the human body.
Medicine Net (2016) defines ‘haematology’ as the diagnosis, prevention, and treatment of diseases related to blood and the bone marrow and that of the vascular system and blood clotting. To them, the study of haematology is as important as it affects the study of many diseases that run in the blood stream. Mandal (2014) on the other hand, puts it that neoplasm is derived from two geek works, ‘neo’, meaning new and ‘plasm’, meaning formation. She, therefore, uses a reference from oncologist Willis to define neoplasm as ‘‘an abnormal mass of tissue, the growth of which exceeds and is uncoordinated with that of the normal tissues, and persistent in the same excessive manner after cessation of the stimulus which evoked the change.’’ Haematological neoplasm is, therefore, a form of cancer of the blood that starts its journey of attack in the cells found in the blood forming tissues like the bone marrow and the cells of body immunity. Categorically, the disease may include leukaemia, multiple myelomas, myelodysplastic syndromes, and lymphomas. From the study researches, chromosomal translocation is said to be the common cause of haematological neoplasm. Further research, as indicated by Rayur (2012), indicates that the disease is rare in children but common in the elderly due to the increase in myeloma with age, mostly affecting those with over 65 years of age. However, notably, within the last decade, the prevention and treatment of the curable diseases under this category has significantly advanced (Bain & Haferlach, 2010).
The signs and symptoms of Haematological neoplasm, as listed in an article written by Cleeland & Williams (2014) include; damage to the bone marrow, anaemia, frequent sickness due to weak white blood cells, general body weakness, fever, fatigue, chills, nausea and night sweats among other symptoms. The main haematological neoplasms include but not limited to; multiple myeloma, non-Hodgkin’s lymphoma, Hodgkin’s disease and myeloproliferative disorder and Chronic lymphocytic leukaemia (Cleeland & Williams, 2014).

A diagrammatic representation of Haematological Malignancies (Rayur, 2012).
Rayur (2012) also notes some of the symptoms of haematological malignancies to be persistent low grade fever, unusual pain resistant to the common therapies, fatigue, unexplained fractures, the occurrence of a typical infection, night sweats, difficulty in breathing and significant weight loss among others (Rayur, 2012).
In order to discover the presence of haematological neoplasm, one needs to undergo a thorough assessment which aims at evaluating the most targeted organs like the heart and the liver. The evaluation should be able to achieve a balance of general condition for the presence of other diseases in the body that is accompanied by this disease, which includes; heart failure and diabetes among other diseases. To an extreme extent, memory loss, nutritional disorder, and depression may be observed. After the assessment, and it a case that the results are positive, treatment always commences immediately. The multidisciplinary teams discuss one’s treatment protocol. Treatment applications like therapeutics procedure based on the resultant diagnosis, medical, staging and social context are applied. At this stage, care during chemotherapy is very important in regulating the blood cells count thus preventing either a rise or a drop in the blood cells (Lloyd-Thomas, Wright, Lister, & Hinds, 1988).
According to a research done by Craig & Foon, (2008) haematological neoplasm does not just attack anyone anyhow. There are certain risk factors that if avoided can reduce the occurrence of this disease drastically. Such risk factors include; the experience of chemotherapy and radiotherapy, accidental exposure to radiation or even worse is the exposure to certain toxic chemicals like fertilizers used everywhere for faming activities and to increase yield, chemical pesticides, and herbicides that may have hydrocarbons and benzene compounds. The occurrence of leukaemia, for instance, is majorly contributed by such risk factors. The biology behind these chemical toxins is that they cause human genes transformation. When these disorders like leukaemia attack the human body, they always affect the functioning of the currently present blood cells and the production of more blood cells thus resulting in low blood cells count and low immunity. It has been reported that most of these haematological neoplasms always starts their development and spread at the bone marrow, an alternative organ for blood formation and production. At the bone marrow, there is always a continued production of three major blood cells; the red blood cells, white blood cells, and the blood platelets. When the neoplasm attacks, it results in the formation of a strange and abnormal blood cell. The abnormally big sized cell normally affects the functioning of the other blood cells thus putting the body at a risk of being attacked by any other disease (Craig & Foon, 2008).
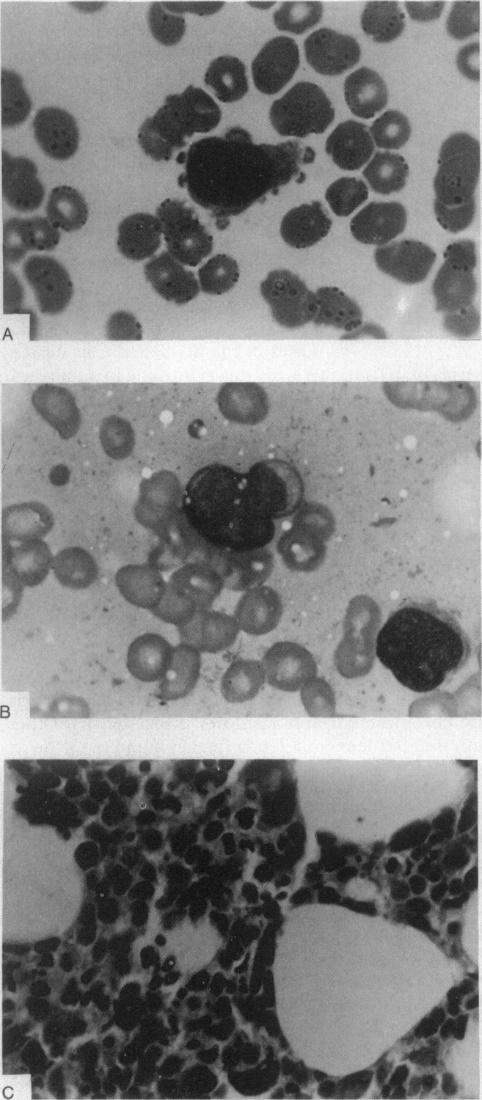
Bone Marrow Specimens from Three Patients with Primary Mediastinal Germ-Cell Tumours and Hematologic Neoplasia ((Nichols, Roth, Heerema, Griep, & Tricot, 1990)
The improvement in innovation and technology has been at the lead in helping control and manage haematological neoplasm. Over the past one decade, there has been a tremendous research work to curb this disease group. The great work is what has led to the development of new techniques that targets both the genetic and the molecular side of haematological neoplasm. Inasmuch as there has not been any cure on the cancerous diseases, some of the recently developed techniques aim at improving the comfortability of the infected thus increasing the chances of survival and the time. What remains a question to be asked is how the patients feel in cases of prolonged therapy. There is needed to keep on exploring much more techniques that will not only prolong the patient’s survival but also make them feel comfortable. This will be possible if further study of each agent is done. With the improvement in innovation and technology towards the assessment and treatment of haematological neoplasm without one having to visit their doctor. A software has been developed, that has a special assessment questionnaire. When a patient starts having some of these symptoms, what they do is that they answer the questionnaire appropriately and sincerely. If the symptoms are very straight and direct, a possible disease is identified. Immediate test then follows. If the results are positive, treatment immediately commences. If a possible disease cannot be identified, then the patient is left with an option of performing a wide test where every possibility is taken into consideration. The advantage of this kind of questionnaire is that it saves on the patient’s energy, time and cost of testing every possibility (Rodriguez-Abreu, Bordoni, & Zucca, 2007).
Because of the wide range of the haematological neoplasm, identification of a single most appropriate disease among them is always slow, tedious, costly and at the same time difficult, notes Nichols, Roth, Heerema, Griep, & Tricot (1990). One approach to identification of the specific disease is the use of the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF). The symptom items in this form were selected from the ones most endorsed at the international standard level and samples of previously and currently infected patients with specifically myeloproliferative neoplasm. Cleeland and Williams (2014) explains that the final MPN-SAF has ten symptoms expected from a myeloproliferative neoplasm. Its ratings are done in the range of zero to ten where zero indicates absence, and ten represents strongly present. The assessment must always be done frequently in order to note the difference after the start of the treatment.
Advances in the Treatment of Haematological Neoplasm
Haematological neoplasm treatment has been revolutionized by the introduction of the monoclonal antibody therapy. This has been made possible by improving the patient’s condition and survival. The global technological improvements are what has driven the sector to identifying better techniques of treatment thus advancing its treatment. The treatment has now become diverse and broader. The introduction of the Imunoconjugates (ICs) is what has made a positive impact in the improvement of the treatment of this disease. It is an advancement that is basically grouped into three; radioimmunoconjugates, also known as the radionuclide, immunotoxins, also known as the protein toxins, and the small molecule drug, also known as the antibody drug conjugates. Before any therapeutic efficacy, it is important that a proper and accurate optimization of the individual components of the Imunoconjugates is done. A recent study conducted by Maria Corinna Palanca-Wessels and Oliver W. Press (2014) indicates that with the introduction of the ICs, treatment of lymphoma has made a great achievement in both multi-agent and monotherapy regimens. The Imunoconjugates comprises a spectacular group of therapeutics that are promising in the management and treatment of haematological neoplasm (Shah, Mehta, Salmon, & Stallard, 2001).
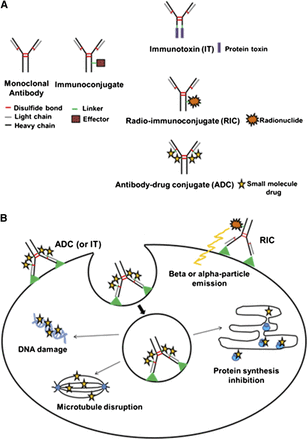
The most challenging part in the treatment of any type of cancer is the difficulty in the administration of a significant dose of tumouricidal agents that help eradicate the diseases found in the human system while at the same time having minimal effects on the normal body tissues. The tumour target delivery can be the most effective method of increasing the quantity of cytotoxic agent that can be safely administered. The development of a therapy with an ability to settle to a malignant cell, based on the surface receptors, was realized with the coming up of the monoclonal antibody therapy. This kind of therapy, although it took over twenty years to get approved, is able to counter deal with most of the haematological diseases. The superiority of this kind of therapy has of course not been an overnight achievement. For instance, the realization that immunoglobulins of murine origin had a high immunogenic effect while at the same time being able to get neutralized by the same tumour immune surveillance system led to immediate tampering with the functionality of antibodies such as the ‘magic bullets.’ The impediment has largely been overcome by the efforts in place that create fully human antibodies. In order to ensure a specific delivery and lethal preload of the cancer cells, the Imunoconjugates must harness the targeting function of the antibodies. This would depend on the availability of a covalently attached effector moiety for the therapeutic purposes. As mentioned earlier, it is this effector that subdivides the Imunoconjugates into three categories, that is, radioimmunoconjugates (RICs), antibody Drug Conjugates (ADCs), and Immunotoxins (ITs) as shown in the figure below;
 Cleeland, C. S., & Williams, L. A. (2014)
Cleeland, C. S., & Williams, L. A. (2014)
The Structure and Mechanism of Action of Imunoconjugates
From the diagram on the previous page, an imunoconjugate consists of an effector molecule, linker, and a monoclonal antibody. In addition, the diagram also shows how the three sub-groups of immunoconjugates are specially linked to different effector molecules. An immunotoxin contains a protein toxin while the radioimmunoconjugate contains a radionuclide. An antibody drug conjugate, on the other hand, carries a tiny drug molecule. Considering the mechanism of action of imunoconjugates, all imunoconjugates has the ability to recognize and bind to a cognate tumour receptor or antigen. For the case of immunotoxins and antibody drug conjugate, there is a need for internalization through a receptor mediated endocytosis in order for it to enter into the target cells. This is followed by a subsequent release of the effector moiety from the imunoconjugate, which takes place through the conditional cleavage of the protease degradation of the antibody within the lysosomal compartment or the antibody. The released drug, what is referred to as the toxin, diffuses into the cytoplasm thus inhibiting the growth of the tumour by disrupting the antibody drug conjugates (ADCs), causing damage to the DNA or ensuring that protein synthesis has been inhibited (Safdar & Armstrong, 2011).
As previously stated, an imunoconjugate consists of three groups; the effector molecule, the target antibody and the linker that joins the effector molecule to the target antibody. Each group plays a vital role towards the success of this kind of therapeutic activity. During the selection of an antibody and an antigen (receptor), several factors have to be considered for its success. An ideal antigen should be expressed at a high level of neoplastic cells. It should be located at the cell surface and its location must have as minimal shedding into its surrounding as possible. The best antibody, on the other hand, should have the ability to penetrate homogenously and quickly to the tumour tissue and be rapidly cleared from the systemic circulation. This should mean encountering as minimal binding to the receptors present as possible. It should not possess an intrinsic antitumor activity as this is done by the effector molecule. Such targets investigated and proven to be ideal include; CD79b, CD22, CD19, CD 30 and CD33 (for the internalizing receptors) and CD45 and CD20 (for the stable receptors (Bernstein, 1997).
To enhance the efficacy of an imunoconjugate, judicious conjugation, modification and selection of the effector molecules is what is needed. A potent effector is important because the cell delivery is limited to the number of surface bounded imunoconjugates. It is said that most effector molecules are usually too toxic that using them without conjugation would mean a failure to get them delivered to the target. To counteract that, synthetic derivatives of the natural compounds have commonly been used. For the case of radioimmunoconjugates, the ionizing radiation affects not only the bound cells but also the neighbouring cells. The use of the alpha-emitting radionuclides that has shorter path lengths and higher energy than the common beta-emitting radionuclides is still under investigation. Studies have shown the application ability of protein engineering to remove the immunogenic sequences from immunotoxins that can generate neutralizing antibodies. To counter the problem of toxicity of the target cell tissues, modification of a drug to a membrane-impermeable form is required. It works by reducing the toxicity stemming from a not specific uptake of unconjugated effector, or alternatively, a premature diffusion and release out of the target cell (Descatha, Jenabian, Conso, & Ameille, 2005).
Li & Zhong (2016) notes that the position of the effector molecules within the antibody and the total number being conjugated can affect antigen binding, clearing, tolerability, potency and sometimes its aggregation. Advances in the technology of linking the antibody and the antigen is what has accelerated the development of potent imunoconjugates. An unstable linker would be one that leads to a non-specific distribution or even rapid clearance which is accompanied by either reduced potency or intolerable toxicity. An ideal linker is one with the ability to prevent the release of premature effectors in the circulation while at the same time allow its liberation in the tumour. The antibody drug conjugates and the immunotoxins are internalized by the receptor –mediated endocytosis, then, transferred to the lysosome. The release of the cytotoxic agent is done conditionally by the cleavable linkers. In order for the cytotoxic agent to be released, there has to be an acid (hydrazine linkage), reducing environment (disulphide bond), and a lysosomal enzyme (peptide bond) available within the endocytic compartment. Difficulties that come with the production of heterogeneous species by traditional chemical conjugation can be overcome by the recombinant DNA technology introduction or modification of the amino acid residues so that it can be able to control conjugation sites.
Illustration by (Cleeland & Williams, 2014)
Radioimmunotherapy (RIT)
Although not very much utilized in the treatment of haematological neoplasm, Radioimmunotherapy has proven its effectiveness in many cases. Inside this technique, the widest clinical experience has been the one involving the RICs that contain beta particles with an isotope emission of (131I or 90yttrium 90Y). This kind of isotope has an added advantage of favourable emission profiles, stable antibody attachment, and its availability. Earlier studies prove that isotope 131I, with a label monoclonal anti CD20, was in the 1990s applied in the treatment of haematological neoplasm. The method worked because of the production of a ‘crossfire effect’ on nearby cancer affected cells. The method could not be able to restrict the production of toxicities in its neighbouring normal tissues. Comparing it with the alpha particles, the alpha particles has a short path length, they exhibit less dependence on energy in order to be able to kill a cell. They, in addition, has a higher linear energy transfer that results in greater cytotoxicity. This suggests that the alpha particles could have been the best to use. However, its utilization in the treatment of haematological neoplasm has not been possible because; they have a short half-life, are limited in availability and they have more difficult radiolabelling in chemistry. That is why they still are not being used in the modern health organizations (Sepkowitz, 2005).
Various studies on radioimunotherapy have been ongoing in order to advance it. Such studies have included studies on setting a Haematopoietic Stem Cell Transplantation (HSCT). By this trial, the scientists are hoping to improve the durability of responses to such treatment. A study done earlier on indicated that using a myeloblative dose of 131I–anti-CD20 RICs, which is approximately five times higher than that of the conventional RIT, followed by an autologous Haematopoietic Stem Cell Transplantation gave an objective remission in about 85% to 95% of the patients infected with multiple relapses. It also indicated a durability of 10 to 20 years remission to about 45% of the infected patients that were involved in the study. This study, together with many ongoing studies has indicated that RIT is the best method that the world currently has. Unfortunately, this technique has not been embraced. The antibody drug conjugate, being the latest, is the one currently being used worldwide in the treatment of haematological neoplasms (Cavallo, 2015).
In conclusion, there has been a tremendous witnessing of the advances in the laboratory diagnosis of haematological neoplasm. Although some have not been embraced, the ones in place have proven to be better than the ones that previously existed. Application of ICs in the treatment of haematological neoplasm, especially RIT, has been very effective even though it is being under-utilized in the modern treatment clinics. There is a lot of ongoing research in the health sector aiming at making better the diagnosis of haematological neoplasm. With such advances, the patients with haematological neoplasm are able to survive for a much longer time.
References
Bain, B. J., & Haferlach, T. (2010). Laboratory Diagnosis of Haematological Neoplasms. Postgraduate Haematology, 395-414. doi:10.1002/9781444323160.ch22
Bernstein, I. (1997). Hematologic malignancies. Current Opinion in Hematology, 4(4), 225-226. doi:10.1097/00062752-199704040-00001
Bourne, M. S., & Cook, T. A. (1965). Haemangiosarcomatosis: Two Cases Presenting as Haematological Problems. BMJ, 2(5456), 275-276. doi:10.1136/bmj.2.5456.275
Cavallo, J. (2015, November 25). The State of Progress in Hematologic Malignancies - The ASCO Post. Retrieved from http://www.ascopost.com/issues/november-25-2015/the-state-of-progress-in-hematologic-malignancies/
Cegolon, L., Mastrangelo, G., & H., J. (2012). Public Health in Primary Care. Primary Care at a Glance - Hot Topics and New Insights. doi:10.5772/37636
Champlin, R. (2016). Relapse of Hematologic Malignancy After Allogeneic Hematopoietic Transplantation. Thomas’ Hematopoietic Cell Transplantation, 836-844. doi:10.1002/9781118416426.ch70
CHEN, C., TSAY, W., TANG, J., TIEN, H., CHEN, Y., CHANG, S., & HSUEH, P. (2009). Epidemiology of bloodstream infections in patients with haematological malignancies with and without neutropenia. Epidemiology and Infection, 138(07), 1044-1051. doi:10.1017/s0950268809991208
Chizuka, A., Suda, M., Shibata, T., Kusumi, E., Hori, A., Hamaki, T., … Kami, M. (2006). Difference between hematological malignancy and Solid tumor research articles published in four major medical journals. Leukemia, 20(10), 1655-1657. doi:10.1038/sj.leu.2404369
Cleeland, C. S., & Williams, L. A. (2014). Symptom burden in hematologic malignancies. Blood, 123(24), 3686-3687. doi:10.1182/blood-2014-03-558981
Craig, F. E., & Foon, K. A. (2008). Flow cytometric immunophenotyping for hematologic neoplasms. Blood, 111(8), 3941-3967. doi:10.1182/blood-2007-11-120535
Descatha, A., Jenabian, A., Conso, F., & Ameille, J. (2005). Occupational Exposures and Haematological Malignancies: Overview on Human Recent Data. Cancer Causes & Control, 16(8), 939-953. doi:10.1007/s10552-005-2301-3
Haferlach, T., & Bain, B. J. (2015). Laboratory Diagnosis of Haematological Neoplasms. Postgraduate Haematology, 332-351. doi:10.1002/9781118853771.ch19
Hamid, G. A. (2012). The Pattern of Hematological Malignancies at Al-Gamhouria Teaching Hospital, Aden, Yemen, from 2008 to 2010. Turkish Journal of Hematology, 29(4), 342-347. doi:10.5505/tjh.2012.03764
Irani, J. L. (2008). Severe Acute Gastrointestinal Graft-vs-Host Disease. Archives of Surgery, 143(11), 1041. doi:10.1001/archsurg.143.11.1041
Kassam, S., Rice, A., Morilla, R., & Bain, B. J. (2007). Case 35: An unusual haematological neoplasm characterized by cells with cytoplasmic tails. Leukemia & Lymphoma, 48(6), 1208-1210. doi:10.1080/10428190701302459
Kassam, S., Rice, A., Morilla, R., & Bain, B. J. (2007). Case 35: An unusual haematological neoplasm characterized by cells with cytoplasmic tails. Leukemia & Lymphoma, 48(6), 1208-1210. doi:10.1080/10428190701302459
Kernbaum, S. (1976). Letter: Intravenous cytarabine in treatment of herpes zoster in haematological malignancy. BMJ, 1(6003), 224-224. doi:10.1136/bmj.1.6003.224
Li, X., & Zhong, H. (2016). The diagnosis, prognosis, and therapeutic application of MicroRNAs in haematological malignancies. Hematology, 21(5), 263-271. doi:10.1080/10245332.2015.1114766
Lloyd-Thomas, A. R., Wright, I., Lister, T. A., & Hinds, C. J. (1988). Prognosis of patients receiving intensive care for lifethreatening medical complications of haematological malignancy. BMJ, 296(6628), 1025-1029. doi:10.1136/bmj.296.6628.1025
Mandal, A. (2014, January 14). Neoplasm Definition. Retrieved February 14, 2017, from http://www.news-medical.net/health/Neoplasm-Definition.aspx
Medicine Net. (2016, May 13). Medical Definition of Hematology. Retrieved from http://www.medicinenet.com/script/main/art.asp?articlekey=22594
Nichols, C. R., Roth, B. J., Heerema, N., Griep, J., & Tricot, G. (1990). Hematologic Neoplasia Associated with Primary Mediastinal Germ-Cell Tumors. New England Journal of Medicine, 322(20), 1425-1429. doi:10.1056/nejm199005173222004
Palanca-Wessels, M. C., & Press, O. W. (2014). Advances in the treatment of hematologic malignancies using immunoconjugates. Blood, 123(15), 2293-2301. doi:10.1182/blood-2013-10-492223
Putti, M. C., & Randi, M. L. (2010). Thrombotic complications in children with haematologic malignacies. Thrombosis Research, 125, S151-S154. doi:10.1016/s0049-3848(10)70034-7
Rayur. (2012, August 12). Hematological Malignancies : Definition, Sign, Evolution, Risk Factor | Rayur. Retrieved February 14, 2017, from http://www.rayur.com/hematological-malignancies.html
Rodriguez-Abreu, D., Bordoni, A., & Zucca, E. (2007). Epidemiology of hematological malignancies. Annals of Oncology, 18(Supplement 1), i3-i8. doi:10.1093/annonc/mdl443
Safdar, A., & Armstrong, D. (2011). Infections in Patients With Hematologic Neoplasms and Hematopoietic Stem Cell Transplantation: Neutropenia, Humoral, and Splenic Defects. Clinical Infectious Diseases, 53(8), 798-806. doi:10.1093/cid/cir492
Sepkowitz, K. A. (2005, April 1). Treatment of Patients with Hematologic Neoplasm, Fever, and Neutropenia | Clinical Infectious Diseases | Oxford Academic. Retrieved February 15, 2017, from https://academic.oup.com/cid/article/40/Supplement_4/S253/437519/Treatment-of-Patients-with-Hematologic-Neoplasm
Shah, S., Mehta, P., Salmon, J., & Stallard, N. (2001). Fiberoptic bronchoscopy and bronchoalveolar lavage in patients with a haematological malignancy with bilateral pulmonary infiltrates. Critical Care, 5(Suppl 1), P040. doi:10.1186/cc1108
Sutcharitchan, P., & Embury, S. H. (1996). Advances in molecular diagnosis of inherited hemoglobin disorders. Current Opinion in Hematology, 3(2), 131-138. doi:10.1097/00062752-199603020-00005
Swansbury, J. (2009). Cytogenetic Studies in Hematologic Malignancies: An Overview. Cancer Cytogenetics, 009-022. doi:10.1385/1-59259-363-1:009
Wajapeyee, N., Wang, S., Serra, R. W., Solomon, P. D., Nagarajan, A., Zhu, X., & Green, M. R. (2010). Senescence induction in human fibroblasts and hematopoietic progenitors by leukemogenic fusion proteins. Blood, 115(24), 5057-5060. doi:10.1182/blood-2009-09-245928



