Master control quality paper
Begin with the website of the Master Control Quality Management System http://www.mastercontrol.com/resource/online_resources.html#od There you will find several demonstrations of how the MCQM system tracks quality while incorporating companywide processes for regulation compliance. Review the 3minute FDA and ISO videos to get familiar with noncompliance risk, software validation, and risk assessment. You do not need to subscribe to the website, but make sure you think of yourself as a manager of IT governance in a big company rather than as a consumer.
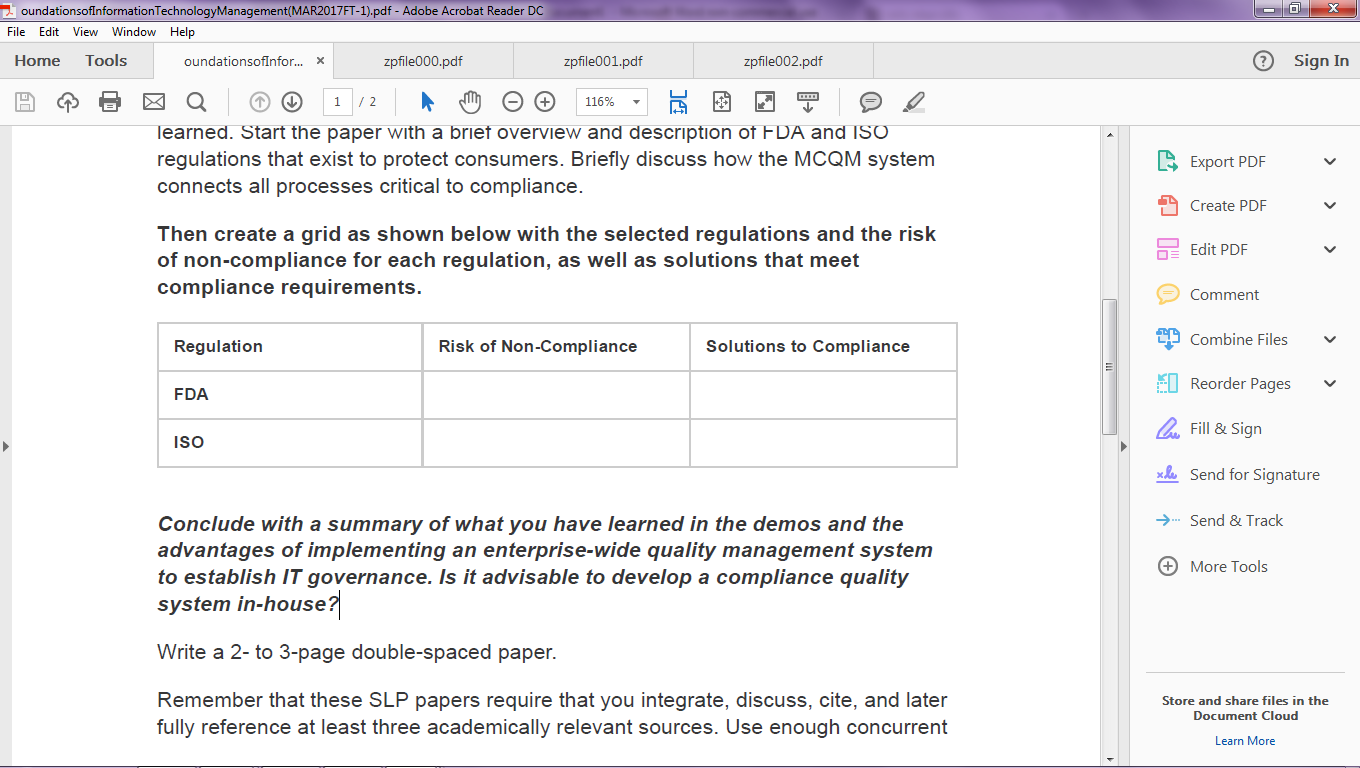
After you review the videos, write a 2 to3 page paper analyzing what you have learned. Start the paper with a brief overview and description of FDA and ISO regulations that exist to protect consumers. Briefly discuss how the MCQM system connects all processes critical to compliance.
Remember that these SLP papers require that you integrate, discuss, cite, and later fully reference at least three academically relevant sources. Use enough concurrent discussion so that the purpose of each citation is apparent. (Note that the paper is incomplete without at least three academically relevant references. Articles such as those in CIO and HBR are acceptable. You may also use military/corporate references, but they do not count as one of the required references.)
1. Demonstrate an understanding of regulations and their impact on IT governance.
2. Demonstrate an understanding of risk for noncompliance.
3. Provide some in text references to modular background readings.



