Weapons of mass destruction unit 6
Explosive Hazards
Bombs do not choose. They will hit everything.
Nikita Khrushchev
Last night the United States dropped four 2000-pound bombs on Saddam Hussein. I don't know anything about explosives, but my Heavens, do those things even need to explode?
David Letterman
INTRODUCTIONIn modern societies the risk of explosive incidents is significant. Numerous businesses manufacture, transport, and employ low and high explosives . in daily operations (i.e., mining and demolition). In addition, explosives continue to be the weapon of choice for terrorists worldwide-of all the weapons of mass destruction (WMDs) (chemical, biological, radiological, nuclear, explosive) reportedly used, the most commonly employed by far, are explosive devices.
Emergency responders encounter explosives in a variety of applications. Military and commercial explosives may be adapted for improvised bombs. In addition, many powerful explosives can be home-made from simple ingre dients. The hazards of explosive weapons are underscored by their relative ease of manufacture; information on bomb making is available in library books and in bomb training manuals available at many gun shows. In add ition, detailed bomb-making instructions are available on the Internet
TERRORISM AND WMDs: AWARENESS AND RESPONSE
DEFINITION OF EXPLOSIVES
An explosive device is any device designed to explode, with concomitant release of a blast wave, gases, light, heat, and sound.
The U.S. Department of Transportation (DOT) defines an explo- sive as
Any substance or article, including a device, which is designed to func tion by explosion (i.e., an extremely rapid release of gas and heat) or which, by chemical reaction within itself, is able to function in a similar manner even if not designed to function by explosion, unless the sub stance or article is otherwise classed. (49 CPR 173.S0(a))
An explosion is defined as the rapid expansion of matter into a greater volume. Conventional high explosives are not the only materials involved in explosions; for example, an industrial boiler can experience a pressure build-up and explode.
The size of an explosion and consequent blast damage will vary as a function of type of explosion (nuclear, mechanical, chemical), type and amount of explosive used, and physical conditions of the materials (e.g., age, whether confined or not).
TYPES OF EXPLOSIONS
Three general categories of explosions exist:
Nuclear
Mechanical
Chemical
A nuclear explosion occurs within the nucleus of an atom as a result of either fission or fusion processes (see Chapter 5). Fission occurs when the nucleus of an atom is split, resulting in the release of massive amounts of energy. Nuclear fusion occurs when light nuclei (e.g., hydrogen or deu terium atoms) are fused, forming a heavier element with the generation of substantial energy. Both reactions have been intensively studied and adapted for use in nuclear weapons. Fission devices were dropped on Hiroshima and Nagasaki, Japan, in 1945. Fusion devices have never been used in warfare.
In a mechanical explosion, the internal pressure of a vessel is greater
that its ability to withstand the pressure. An example is the bursting of a pressure cooker.
EXPLOSIVE HAZARDS
A chemical explosion results from the ignition of an energetic (react ive) substance. A chemical disintegration occurs, along with the produc tion of light, heat, and a shock wave. Chemical reactions take place within the electron cloud of an element; this is also where an explosion occurs. This chapter will be devoted solely to chemical explosions.
HISTORY OF EXPLOSIVES DEVELOPMENT
Ancient Times to the Renaissance
Among the first of all energetic (explosive) materials ever developed and tested was black powder. Historians, however, still differ regarding its origins; the Chinese, Hindus, and Arabs have all been credited with its discovery. In 1200 CE, Arabian Abd Allah recorded the. use of saltpeter as a main ingredient of black powder. In 1249, Roger Bacon, an English monk, documented a formula for black powder that included saltpeter, charcoal, and sulfur.
Early in its development black powder was found to be useful for both military and work purposes. The Chinese and Europeans became aware of the benefits of black powder at about the same time. By 1232 the Chinese developed rockets and a weapon they called "heaven-shaking thunder crash bomb," an iron bomb attached to a chain that could be low ered from city walls to explode among attacking forces (Langford, 2004; Meng, 2005). The earliest mention of black powder on military supply lists was in 1326.
The explosive mixtures in use to this point were for black powder, not gunpowder. Different proportions of saltpeter, charcoal, and sulfur were required to fire weapons from the early cannons. In the mid-12th century, John Arderne, an Englishman, gave the proportions of saltpeter, charcoal, and sulfur as 6:2:1. The Germans used a mixture of 4:1:1 in 1350. Other, mostly useless ingredients such as wine, urine, arsenic, amber, alcohol, and camphor were added to the recipe to improve stability, reduce absorp tion of moisture, and prevent crumbling.
In the 17th and 18th centuries a number of advancements in explo sives manufacture occurred. In 1654, ammonium nitrate was prepared by J.R Glauber, a German chemist. Picric acid, a highly unstable and powerful explosive, was discovered by Pierre Woulfe, a French chemist in 1771 by treating silk with nitric acid. Soon afterward potassium chlor ate was prepared by Claude Berthollet, a French chemist. His proposal to
TERRORISM AND WMDs: AWARENESS AND RESPONSE
use potassium chlorate instead of potassium nitrate in black powder was dropped after a catastrophic explosion during manufacture in 1788 (U.S. Army, 1990).
Nineteenth CenturyBy the 19th century modern energetic materials technology had arrived. New explosives were discovered, replacing the black powder mixtures that had been so popular for weapons and blasting for centuries. New energetic materials influenced the design and performance of weapons; concomitantly, the invention of new weapons prompted the search for more effective and less expensive explosives.
In1800, Edward Howard, an English chemist, discovered mercury ful minate, a highly sensitive and powerful explosive. In 1807, the use of mer cury fulminate as an explosive primer was patented by the Scot Alexander
Forsyth. In 1825, Rev. Dr. Clayton in England isolated benzene, creosote,
and naphthalene from coal tar. These products were later incorporated into explosives such as trinitrobenzene and nitronaphthalene.
The application of a flame directly to explosive solids (e.g., in mining) was a highly dangerous activity. In 1831 the safety fuse was invented by British engineer William Bickford. Theso-called Bickford fuse contained a core of black powder tightly wrapped in jute yarn. The fuse was designed to ensure accurate and consistent burning time. The Bickford safety fuse was later made waterproof by applying a coat of asphalt covered with textile.
Nitrostarch was discovered in 1833 by Henri Braconnot, a French
chemist, while dissolving starch in concentrated nitric acid (Labrude and Becq, 2003). In 1845 guncotton (later termed nitrocellulose) was formulated by Christian Schoenbein, a German chemist at the University of Basel, Switzerland, by treating cotton with a nitric acid-sulfuric acid mixture. Schoenbein demonstrated that nitrocellulose was up to four times as powerful as black powder for blasting. During early efforts to manufac ture guncotton serious accidental explosions occurred, which delayed its general use as an explosive. Eventually, however, guncotton became uni versally accepted for use in blasting.
Nitroglycerin, an ester of glycerin and nitric acid, was invented by Asconio Sobrero, an Italian chemist, in 1846. Nitroglycerin was found to be extremely sensitive to even slight shocks, so its commercial use was delayed. This promising explosive was eventually used on a large scale following the invention of dynamite, blasting gelatin, and smokeless
EXPLOSIVE HAZARDS
powder. Nitroglycerin production facilities were designed for commercial application by Alfred Nobel, a Swedish chemist (Figure 6.1). In 1862, the first plant was constructed at Heleneborg, Sweden.
In 1863, trinitrotoluene (TNT) was prepared by J.Willibrand, a German
scientist. TNT had been used for many years in the dye industry; however, it was not used as an explosive until 1904. Thereafter, TNT became one of the most commonly used high explosives. Two years later nitrocellulose was purified by Sir Frederick A. Abel, an English chemist. Abel pulped, washed, and compressed nitrocellulose into blocks, sheets, disks, and cyl inders. These forms were found to be very useful for rock blasting.
In 1866, dynamite was invented by Alfred Nobel by absorbing nitro glycerin (75%) in kieselguhr (diatomaceous earth) (25%). Kieselguhr, an inactive ingredient, stabilized the nitroglycerin, and made dynamite a much safer explosive to handle. Dynamites with an "active base" were soon patented by Nobel. In these new formulations nitroglycerin was mixed with combustibles (sawdust, charcoal, starch, etc.), and oxidizers (sodium nitrate or potassium nitrate). By 1884 ammonium nitrate became widely used in dynamite formulations. These additives resulted in a more efficient explosive than earlier dynamite recipes. In 1868, Nobel went on to invent the blasting cap, a device used to initiate larger explosives.
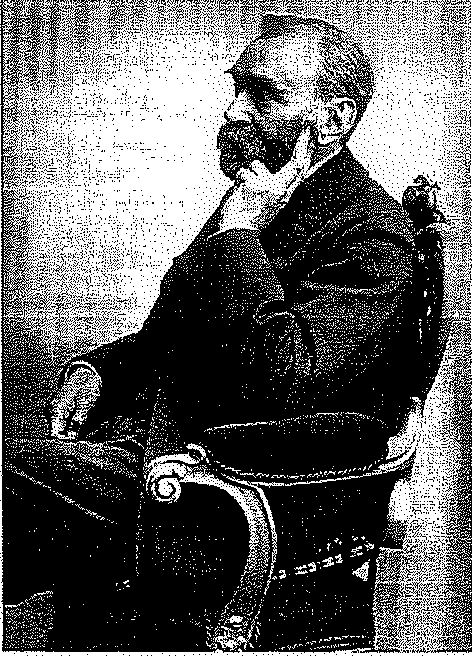
Figure 6.1 Alfred B. Nobel, Swedish chemist and inventor of dynamite.
TERRORISM AND WMDs: AWARENESS AND RESPONSE
Nobel's cap consisted of mercury fulminate in a copper tube. The cap was crimped to one end of a safety fuse and then inserted into the dyna mite casing.
In 1875, the Explosives Act was enacted by the British Government after a catastrophic explosion in Birmingham killed 53. The Act called for trained inspectors, who were given the power to inspect all factories and storage magazines to ensure that manufacturing, storage, and handling operations were carried out safely. As a result, the number of deaths in explosives factories was greatly reduced.
Smokeless powder was invented in1884 by Paul Vieille of France. This powder proved valuable on the battlefield, as the enemy was no longer capable of locating a hidden shooter by the smoke of his gun. The shaped charge was discovered accidently in 1888 by Charles Munroe, a scientist at the U.S. Naval Academy. Munroe discovered that a concave-shaped explosive charge was capable of piercing a steel plate. Munroe's discov ery went on to have important benefits to both military and industrial applications.
In 1889, cordite was prepared by Dr. W. Kellner. Cordite was patented for the British Government and wasadopted asa military propellant named CSP (cordite smokeless powder). The high explosive cyclonite (RDX) was first prepared in 1899 by Georg F. Henning of Germany. Starting in 1925 RDX was manufactured at in industrial scale at Picatinny Arsenal, NJ. It was not until World War II, however, that the value of RDX as an explosive truly came to be appreciated. Extensive research into RDX manufacturing processes and applications were carried out at this time. Pentaerythritol tetranitrate (later to be termed PETN) was first synthesized in 1894 by nitration of pentaerythritol (Zoltan, 2007).
At the end of the 19th century aluminized explosives, containing aluminum to increase performance, were first proposed in Germany. Ammonal, the first such explosive, contained ammonium nitrate, char coal, and aluminum. It was not until World War II, however, that the United States and Allied forces realized the benefits of aluminized explosives.
In the early 20th century antifreeze for dynamite was researched extensively in many countries, in part as a result of a disastrous explosion during defrosting dynamite in Germany. Sigurd Nauckhoff of Sweden published his work on dynamite antifreeze in 1905, listing requirements for satisfactory antifreeze, and suggesting compounds that met these requirements (Fordham, 1980). In the same year nitrostarch was produced in a stable form. Nitrostarch is chemically similar to nitrocellulose but is
EXPLOSIVE HAZARDS
lower in strength. Nitrostarch does not cause the health effects from skin contact as do TNT, nitroglycerin, and dynamite.
Early in the new century, great strides were made in development of detonating cord and blasting caps. In 1908, a hollow cord containu1g TNT instead of blackpowder was patented in France. This detonating cord had a detonation velocity of 4880 m/s (16,000 feet per second [fps]). In 1914, lead styphnate (trinitroresorcinol), an initiating explosive, was first pre pared by E. von Herz of Germany. Russian Col. AA Solonina was the first to propose using lead styphnate in detonators. Ammonium picrate (Dunnite or Explosive D) was standardized in the United States as a bursting charge for armor-piercing shells. These projectiles could be fired through 12 inches of armor plate, and could be detonated on the far side by an insensitive primer. By 1912, TNT was adopted as the standard burst ing charge in high-explosive shells for the field artillery of the U.S. Army.
World War I
At the outbreak of war in the summer of 1914, the German General Staff had planned to fight a conflict fueled predominantly by high explosives. The Germans rapidly converted their chemical industrial facilities to pro duction of synthetic ammonia, nitric acid, and sulfuric acid, all required for the manufacture of explosives and chemical warfare agents. The key to German explosives production was the Haber process for producing ammonia from atmospheric nitrogen. Using the Haber process Germany became independent of foreign nations for its supply of ammonia and nitric acid.
Smoke munitions were used on land and sea by the belligerent pow ers in World War I. In July, 1915, the British used "smoke pots," vessels filled with pitch, tallow, black powder, and potassium nitrate. The first large-scale smoke operation occurred on September 20, 1915, when the Canadians fired several thousand smoke shells from trench mortars dur ing the attack against Messines Ridge (Belgium).
About 2,500,000 tons of high explosives were used by the warring powers during the war, resulting in an estimated 10 million casualties.
Interwar Years
During the period between the two world wars, the U.S. Army invested significant funding and resources toward the formulation of ammu nition and weaponry. During these two decades RDX, PETN, ethylene
TERRORISM AND WMDs: AWARENESS AND RESPONSE
dinitramine (EDNA), lead styphnate, and lead azide were developed as military explosives. The development of processes for producing toluene from petroleum eased limitations on availability of TNT, and permitted development of powerful and castable explosives like composition-B and pentolite (U.S. Army, 1990). During World War I the supply of toluene was quite limited-it was derived primarily from coal as a by-product of coke ovens, and some was extracted from natural gas.
From the 1920s onward, scientists at Picatinny Arsenal (NJ) had been searching for a compound having the high brisance (shattering power) of RDX without the sensitivity to friction and impact. Research by chemist George C. Hale and others led to the discovery of ethylenedinitramine (EDNA), the first entirely American high explosive. More powerful than TNT, EDNA was slightly less powerful than RDX but was also less sensi tive. EDNA's stability gave it an important advantage in terms of manu facturing, loading, storage, transportation, and field use. Named haleite (in honor of Dr. Hale) this new explosive could be loaded into small shells without a desensitizing agent.
World War IIWhen World War II began in September 1939, the standard United States charge for high-explosive bombs was TNT. Early in the war, however, other fillings were used, such as RDX, an explosive known for its great power and brisance but generally considered too sensitive. The British developed a method of using beeswax to desensitize the RDX, and used this explosive with success in the 2-ton "blockbuster" bombs dropped on Berlin in April 1941. The most sensitive of all high explosives was PETN, which was even more readily detonated than RDX. PETN was extremely sensitive to shock; therefore, it was diluted with TNT to desensitize it. The new composition, named pentolite, has been extensively used in detona tors, bazooka rockets, rifle grenades, boosting devices, and in the shaped charges of anti-tank shells (U.S. Army, 1990).
Owing to the extensive use of armor in World War II, a great deal of research was devoted to development of anti-tank weapons, armor piercing shells, and shaped charge munitions. New special-purpose bin ary explosives, such as tetrytol and picratol, were developed for use in demolitions, chemical bombs, and armor-piercing weapons. A number of plastic explosives used for demolition work were developed in Great Britain and the United States, the most important being the C-3 compos ition based on RDX.
EXPLOSIVE HAZARDS
Primacord, a highly accurate detonating cord, was created in 1936 by the Ensign-Bickford Company, CT. Primacord consisted of PETN covered with textiles and waterproofing material. This detonating cord had a vel ocity of 21,000 fps (6405 m/s), and has been used extensively by the armed forces for demolition work.
The development of napalm ("nap" for napthenic acids; "palm" for the coconut fatty acids that comprised the thickener) by the United States in 1941 made use of gasoline in flame weapons. This gelled mixture made it possible for aircraft to deliver firebombs over difficult areas. Napalm proved to be one of the most effective aerial incendiary agents in the war. On the evening of March 9, 1945, more than 300 B29 bombers flew over Tokyo, dropping about 2000 tons of incendiaries, mostly clusters of M69 6-pound bomblets. The M69 was a tube that contained a black powder propellant charge that ignited and projected the napalm filling from the tube (U.S. Army, 1990). Aerial photos indicated 16 square miles had been destroyed by fire. Records examined after the war showed that more than 250,000 buildings in Tokyo were destroyed in this raid. More than 100,000 tons of incendiaries were dropped on the cities of Japan during World War II. Most of these were M69 bomb clusters.
Post-World War IIDue to United States involvement in the Korean War (1950-1953) and the Vietnam Conflict (1964-1973), there was an incentive for development of even more effective munitions and more powerful explosives (Figure 6.2). In 1950, MOX (metal oxidizer explosives) were developed by the National Fireworks Ordnance Corporation, West Hanover, MA, for use mostly in small caliber anti-aircraft shells.
In 1952 plastic-bonded RDX was developed by the Los Alamos Scientific Laboratory of the University of California for use as a mechan ical strength explosive-this new material was useful in hardening man ganese alloys, among other applications. PB-RDX consisted of 90% RDX, 8.5% polystyrene, and 1.5% dioctylphthalate. By 1960, detacord, detaflex flexible cord explosive, and detasheet flexible sheet explosives were devel oped by the DuPont Company.
Slurry explosives were developed by M.A. Cook and H.B. Farnham by adding water to ammonium nitrate to form slurries of the oxidizer salts, ammonium nitrate and sodium nitrate, and a solid fuel sensitizer. Slurry explosives were found to provide many times the detonation force of ammonium nitrate-fuel oil (ANFO) explosives.
TERRORISM AND WMDs: AWARENESS AND RESPONSE
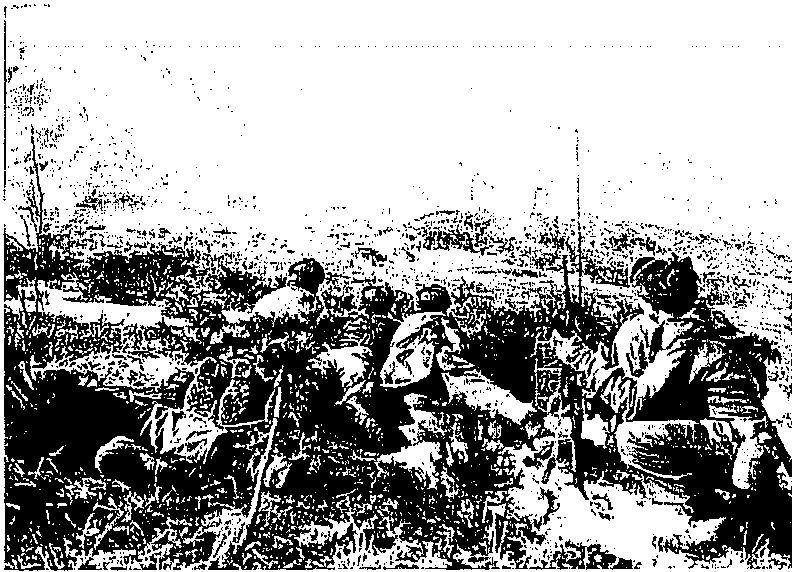
Figure 6.2 An artillery officer directs troops as they fire white phosphorous shells on a Communist-held post in February 1951. (http://www.archives.gov/ publications/prologue/images/korean-war-artillery.jpg.)
In 1992, in the aftermath of the Gulf War, explosives were used to extinguish most of the 700 Kuwaiti oil well fires that had been set by Saddam Hussein's retreating troops. One method involved the placement of high explosives in augered shafts at inclined angles to the well pipe. These explosives remove debris to create a ramp for access to the well pipe and also crimp the well pipe. Two bombs may be physically placed such that they straddle the well. The bomb may be air dropped or physically placed in position (Freepatentsonline, 2009).
CHARACTERISTICS OF EXPLOSIVESFor a chemical to be considered an explosive, the material must exhibit the following (NATO, 1996):
Initiation of reaction. A reaction must be capable of being initiated by the application of shock, heat, or similar insult to a small portion of the material.
Rapidity of reaction. An explosive reaction is distinguished from a typical combustion reaction by the great speed with which
238
EXPLOSIVE HAZARDS
it occurs. Unless the reaction occurs rapidly, the thermally expanded gases will be dissipated in the medium, and there will be no explosion.
Formation of Gases. Gases are evolved from the decomposition of solid or liquid fuels. When a carbonaceous substance is burned, the carbon and hydrogen in the fuel combine with oxygen in the atmosphere to form carbon dioxide and H2O (steam), together with flames, heat, and light.
Evolution of Heat. The generation of large quantities of heat accom panies every explosive chemical reaction. This rapid liberation of heat causes the gaseous products of the reaction to expand and generate high pressures. Liberation of heat at slow rates will not cause an explosion.
CLASSIFICATION OF EXPLOSIVESThe U.S. DOT regulates the transportation of chemical explosives and articles containing explosives. DOT recognizes five divisions of Class 1 (explosives): 1.1 through 1.5 (Figw·e 6.3). These divisions are explained in Chapter 3.
(a)
(c)
Figure 6.3 Selected DOT placards for an explosive article.
239
TERRORISM AND WMDs: AWARENESS AND RESPONSE
A number of other systems are available for classification of energetic materials; for example,
9 Pure explosive chemicals versus mixtures
Chemicals versus use forms (slurries, emulsions and sheet explosives)
Chemical class (nitroamines versus nitroaromatics versus nitrate
esters)
Rate of decomposition (low explosives versus high explosives)
Commercial versus military types
In thissection we will discuss explosives types based on rate of decom position, military versus commercial materials, and use forms. Following this will be a discussion of improvised explosive devices (IEDs).
Rate of Decomposition
Explosives are classified as low or high explosives as a function of rate of decomposition. Low explosives burn rapidly (or deflagrate), while high explosives detonate (Box 6.1). This classification depends, in part, on how the materials are packaged and used. One of the most important proper ties involved in rating an explosive is detonation velocity, a value desig nated for both confined and unconfined conditions. Detonation velocity is the speed at which the detonation wave travels through the explosive. This property is usually measured in an unconfined column of explo sive 1¼ inches in diameter. Detonation velocity is a function of chemical
BOX 6.1 BASIC TERMINOLOGY ASSOCIATED WITH EXPLOSIVES
Deflagration: Rapid burning with the generation of intense heat and light. This reaction occurs at subsonic speeds (i.e., less than 1130 fps) (343 m/s). Deflagration typically occurs with pyrotechnic materials as well as propellants such as smokeless powder and black powder. Detonation: An explosive event of almost instantaneous decom position of fuel. Detonation reactions occur at supersonic speeds (greater than approximately 1130 fps). Detonation is a form of energy
release that supports a shock wave.
Deflagration to Detonation Transition (DDT): The transition from deflagration to a detonation, resulting in an explosion.
240
EXPLOSIVE HAZARDS
composition of the explosive, density, particle size, charge diameter, and degree of confinement. Factors that increase detonation velocity include decreased particle size, increased charge (i.e., fuel) diameter, and increased confinement. The confined detonation velocity of commercial explosives varies from about 4000 fps to 25,000 fps (Table 6.1.) (Global Security, 2008).
Low ExplosivesLow explosives function by deflagration (i.e., rapid combustion). Shock waves of low explosives travel at speeds less than 1130 fps. Low explosives are normally employed as propellants and pyrotechnics, and include black powder and mostsmokeless powders. Both black powder and smokeless powder are employed in both military and civilian applications.
Propellants
Propellants are designed to release a gas under controlled conditions to perform useful work functions. Examples include the deflagration of gun powder for firing a projectile (e.g., bullet) out of the barrel of a gun. The key to safe and effective use of propellants is to maintain a controlled release of gas. When propellants are confined or when control is lost, explosions may occur.
Pyrotechnics.
Pyrotechnics are designed to produce light, smoke, heat, and sound. The pyrotechnics we are most familiar with include fireworks, which are designed for entertainment. However, pyrotechnics are also important in the workplace, for example, as ingredients of road flares and other illu mination devices.
High ExplosivesWhile propellants or low explosives are designed to produce a con trolled release of energy, high explosives are engineered to detonate with a near-instantaneous release of energy. High explosives func tion by detonation: shock and heat waves travel at speeds greater than 1130 fps. Some energetic materials have been measured to attain speeds over 29,000 fps. Because of their destructive force, high explosives are employed in military ordnance. High explosives are usually initiated by a blasting cap but high temperatures, shock, or friction may also cause initiation.
241Table 6.1 Chemical and Physical Properties of Selected Explosives >-J
"'
:-<l
Rate of Detonation Chemical :-<l
Classification Explosive (fps) Formula Density Sensitivity
Low explosives Black powder 1312 KNO3, S, C Varies Low :,,.
Smokeless powder Rapid burning Varies Low Primary Lead azide 13,400-17,000 Pb(N3h 4.71 g/ cm3, solid High explosives Lead styphnate 17,100 C6HN3O 8Pb 3.02 g/ cm3, solid High
Mercury fulminate 11,500-21,100 Hg(CNO), 4.43g/cm3 High [;?
:,,.
Secondary Ammonium picrate 22,500 C,H2(NO 2),ONH 4
explosives C-4 26,400
"'
Flex-x 22,300 "z'
HMX 29,900 Csflz(NO2)aONH• 1.91 g/ cm3, solid Low "{J) '
{J)
N :,,.
"N" Nitrocellulose 21,900 z
Nitroglycerin 4900-25,400 C3HsN3O 9 1.6 g/ cm3 at15 °C High tJ
PETN 27,200 C5H8N4O12 1.77 g/ cm3 at 20 °C Medium ""''
{J)
Picric acid 19,000 C6H3N 3O 7 1.76 g/ cm3, solid 'u
RDX 26,800 C3H 6N,O 6 1.82 g/ cm3 Low
Tetryl 25,800 C7H5N5O, 1.73 g/ cm3 Insensitive "'
llinitrotoluene (TNT) 21,800-22,400 C1flsNaO, 1.65 g / cm3 Insensitive
Tertiary Ammonium nitrate 3300-8200 NH 4NO3 1.72 g/ cm3, solid explosives
Sources: Beveridge, A. (ed.), Forensic Investigation of Explosions. CRC Press. Boca Raton, FL. 8, 1998; U.S. Department of the Army. Military Explosives. Technical Manual. TM 9-1300-214. Washington, DC, 1990; U.S. Department of the Army. 1971. Explosives Series. Properties of Explosives of Militan; Interest. Engineering Design Handbook. Washington DC. January, 1971.
EXPLOSIVE HAZARDS
High explosives are divided into three classes: primary, secondary, and tertiary, based on their sensitivities to various stimuli. Primary explosives are the most sensitive and tertiary the least sensitive. For first responders attempting to identify the safest course of action in a hazardous situation, understanding sensitivity of an explosive material is critical.
Primary Explosives ·
Primary explosives are all extremely sensitive and therefore extremely dangerous to handle and transport. Some important primaries are pre sented in Table 6.2.
The U.S. military had attempted to use a number of primary explosives for conventional munitions; however, due to their extreme sensitivity such use was abandoned. Also as a result of their sensitivity, primary explosives are typically used in only small quantities, for example, in blasting caps. Primary explosives are therefore useful in initiating less sensitive, but more powerful secondary explosives in a so-called firing train (Box 6.2).
Secondary Explosives
Secondary explosives are markedly less sensitive than primary explosives and require a significant stimulus (e.g., the energy created by another explosion) to detonate; therefore, secondary explosives are initiated by a primary explosive. Secondary explosives are most commonly used in bulk quantities. Some popular examples include the following:
Trinitrotoluene (TNT)
Pentaerythritol tetranitrate (PEIN)
Cyclotrimethylene trinitramine (RDX)
Cyclotetramethylene-tetranitramine (HMX) (Box 6.3)
Table 6.2 Some Common Primary Explosives
Copper acetylide Nitrogen triiodide
Hexamethylene triperoxide diamine Silver acetylide Lead azide Silver fulminate
Lead picrate
Lead styphnate Mercury fulminate Nitrogen trichloride
Silver azide
Tetramine copper complexes Tetryl (2,4,6-trinitrophenyl-N- methylnitramine)
Triacetone triperoxide
TERRORISM AND WMDs: AWARENESS AND RESPONSE
BOX 6.2 PRIMARY EXPLOSIVE: LEAD AZIDE
Lead azide is the most popular primary explosive in use today (Figure 6.4). It is rated as highly sensitive to both friction and shock. The velocity of detonation is approximately 17,500 fps. Its color var ies from white to buff. Lead azide is widely used as an initiating explosive in high-explosive detonator devices. Because lead azide does not react with aluminum, detonator capsules for lead azide are composed of this metal. The hygroscopicity of lead azide is very low, that is, it does not attract water. Water does not reduce its impact sen sitivity, as is the case with other primaries like mercury fulminate. Lead azide is completely stable in storage.
Lead azide is poisonous to biota (Global Security, 2008).
Figure 6.4 Structure of lead azide.
Secondary explosives are manufactured to tolerate rough handling.
Regardless, however, they must still be handled with extreme caution.
Tertiary Explosives
Tertiary explosives are the most insensitive of the high explosives. Most formulations contain the common salt ammonium nitrate. Ammonium nitrate-fuel oil (ANFO) is a common tertiary explosive and a very insensi tive substance. ANFO is discussed later in this section. Tertiary explo sives require a significant stimulus to cause detonation. A blasting cap, shock, or small flame will not initiate them. Usually a quantity of second ary explosive (a booster), for example, dynamite, is needed for initiation.
The Firing TrainIn military and commercial explosives as well as in terrorist weapons, a sequence of primary, secondary, and often tertiary explosives is employed for maximum effectiveness. The firing train (also known as the explo sive train) is a specific sequence of steps resulting in progressively larger
EXPLOSIVE HAZARDS
BOX.6.3 SECONDARYEXPLOSIVE: HMX
HMX, also known as octogen . and cyclotetramethylene tetranitrarnine, is a powerful and relatively insensitive nitramine high explosive which is chemicall:l;' related to RDX (see below). The "HMX" acronym has beenassigne1 a variety of names, for example,
. "high melting explosive" and "her majesty's explosive':; however, the accepted name is "high-molecµlarcweight RDX"(Figure 6.5).
HMX is the highest energysCllid explosive produced on a large
·scale in the United States,Itexpl()des violently athightemperatures·· (534°F and above). Because oHhis'property, HMX is used exclusively for mi!ita1·y purposes asa co!Ilponent of>flastic-bonded explosives,
.· of rocket propeHant a1;1d hightxplos}y . burst r charge.s, and to
.·irnploc:le..•fjssionat+e .·•mNe.i::f\lI/11. rmclearcleyices,.•(Gloqal, Se. J.1rityi 2pp }. [IM)( is selc:!Cl!)l}'.: ep.:.alone in mHit rY applkatiops;lmt i .typ-; ically I11i;<:ed with otl:le1 f o.g;ppunds such s Jl\IT,
-o +... o
'N'
I
O ,.---N----. o-
'\ + I \ ...
N-N N-" ·
-o' \. ,J 'b
N .
I
<YN::O_Figure 6.5 Structure of HMX.
explosions. The firing train explosions increase in size until the main charge is detonated (Figure 6.6).
A minor stimulus begins the train. The stimulus can be fire, an electric pulse or other insult. If the stimulus is a flame, this will ignite a propel lant (e.g., black powder contained within a time fuse). The burning pro pellant delivers flame to a primary explosive or detonator contained in a blasting cap. The primary explosive immediately detonates, delivering a shock and flame to the secondary explosive. The secondary explosive or booster immediately detonates, delivering a significant shock to the tertiary explosive. The tertiary explosive or main charge then detonates, completing the firing train.
Other possible firing trains, which vary in complexity, can be devised. For example, for black powder confined within a steel pipe in an !ED, only
TERRORISM AND WMDs: AWARENESS AND RESPONSE
| Stimulus | Igniter | Detonator | Booster | Main |
| (Insult) | charge |
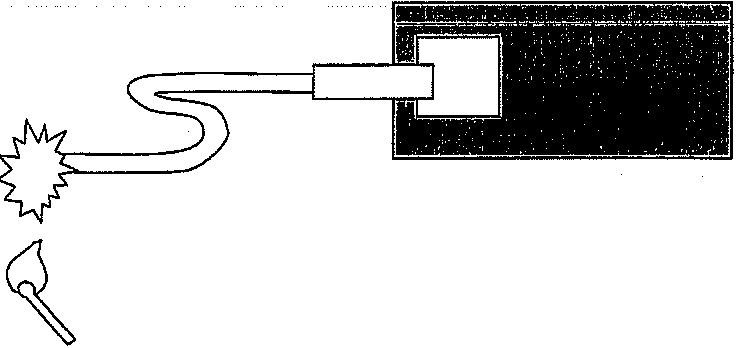
Flame --+ Time fuse - Prlmary - Secondary -Tertiary or explosive explosive explosive
Det cord
Figure 6.6 Schematic of an explosive firing train.
three steps are needed: (a) a flame ignites a fuse, (b) the fuse ignites the black powder inside the pipe, (c) a deflagration to detonation transfer (DDT) and explosion occurs.
COMMERCIAL AND MILITARY EXPLOSIVESMilitary explosives are designed to deliver an enormous amount of energy very quickly. This ability also allows them to effectively shatter or cut tar gets. Many commercial explosives have the same characteristics as mili tary explosives; however, numerous commercial explosives are designed to push or heave a target, rather than shatter.
Commercial ExplosivesBlack Powder
Black powder is composed of 75% potassium nitrate (saltpeter) or sodium nitrate, 10% sulfur, and 15% charcoal, a recipe essentially unchanged since its discovery by alchemists in the 13th century. Black powder mix tures range in color from black to dark brown, and grains range in size from fine powder to large granules ("prills"). The burning speed of black
EXPLOSIVE HAZARDS
powder is controlled by granule size (i.e., surface area)-the smaller the granules, the more rapid the burn rate.
Black powder is used as a propellant in weapons such as muzzle loading rifles and cannons. Black powder is also used as the burning com ponent in safety fuses as well as in some pyrotechnic fireworks and hobby fuses.
Black powder is readily ignited by a spark or squib (a small explosive device); powder fuse, or primer in shotgun cartridges. When initiated, black powder deflagrates; it does not detonate. The rapid burning is an
oxidation-reduction reaction (Meyer, 2004):
32KN03<sl + 3Ss,,i + 16C(,J -, 16 K2C0 3(J, + 16N 2 (lg + 24S0 2(lg (6.1)
The burning phenomenon occurs so vigorously that it may resem ble an explosion. Black powder is such a reactive formulation that it should be stored, transported, and handled as if it were a high explo sive. Black powder is indeed classified as a high explosive under DOT regulations. Packages of granular and compressed black pow der are labeled EXPLOSIVE l.lD; transport vehicles are placarded EXPLOSIVES 1.lD.
Black powder does not deteriorate with age. Water may temporarily desensitize it; however, once dry, it regains its original reactive compos ition. Black powder is extremely sensitive to friction, flame, impact, shock, and static electricity. This characteristic makes black powder one of the most dangerous explosives to handle.
Smokeless powder (Figure 6.7) has replaced black powder in many applications. The term "smokeless" has been used because its combustion products are mainly gaseous, compared to more than 50% solid products for black powder.
Nitroglycerin
Nitroglycerin (1,2,3-trinitroxypropane or glycerin trinitrate) is an oily, pale yellow liquid high explosive (Figure 6.8).
For well over a century, nitroglycerin has been a key ingredient in the manufacture of explosives, specifically dynamite. As early as the 1880s, nitroglycerin was adopted as a military propellant for use in rifles. Nitroglycerin continues to be used in military weapons, as a gellatinizer for nitrocellulose, and as an ingredient in some solid propellants. Nitroglycerin is a popular component of explosives used in the construction and
TERRORISM AND WMDs: AWARENESS AND RESPONSE
Figure 6.7 Smokeless powder sold as commercial product. (MAGPRO. Reproduced with kind permission of Western Powders.)
Figure 6.8 Structure of nitroglycerin.
demolition industries. Nitroglycerin also has medicinal uses; it has been used for treatment of heart and certain blood-circulation diseases.
Nitroglycerin is obtained by the nitration of glycerol. Glycerol is added to a mixture of nitric acid and sulfuric acid:
CH20 H CH20 - N O,
CHOH+ 3HN0 3 --, CHO -NO,+ 3H20
I ICH 20 H CHO-NO,
(6.2)
EXPLOSIVE HAZARDS
Sulfuric acid serves as a catalyst in the above reaction.
Nitroglycerin is one of the most powerful explosives, with blast effects comparable with those of RDX and PETN (discussed below). Nitroglycerin is extremely sensitive to shock; even slight physical jarring can cause it to initiate. In addition nitroglycerin decomposes over time to even more unstable forms. Nitroglycerin is, ther.efore, extremely dangerous to use or even transport. Due to its high degree of sensitivity (Table 6.1), nitrogly cerin is rarely used alone as an explosive. As a result of numerous cata strophic accidents (Central Pacific, 2005), liquid nitroglycerin has been widely banned. More stable explosives such as dynamite and related mix tures have been prepared by mixing nitroglycerin with inert absorbents (diatomaceous earth, sawdust, etc.).
According to U.S. DOT regulations nitroglycerin is a forbidden explosive. However, when desensitized, DOT regulates the transpor tation of liquid nitroglycerin as a division 1.1D explosive (49 CFR, 2007).
Nitroglycerin is toxic. by ingestion, inhalation, and skin absorption. It causes dilation of small veins, capillaries, and coronary blood vessels, thus leading to severe headaches and decreased blood pressure. After repeated nitroglycerin exposure, tolerance to this vasodilatory activity will even tually occur. Chronic repeated exposures to nitroglycerin, however, have been associated with more serious cardiovascular effects including angina pectoris and sudden death (Global Security, 2008).
Dynamite
Dynamite consists of three parts nitroglycerin, one part diatomaceous earth (i.e., a naturally occurring, chalk-like sedimentary rock) and a small amount of sodium carbonate. This mixture was formulated in 1867 by Alfred Nobel, who was concerned with the sensitivity and occupa tional hazards of nitroglycerin. He discovered that nitroglycerin could be absorbed into a porous material and become much safer to handle than liquid nitroglycerin alone. As noted above, nitroglycerin is highly shock-sensitive; however, adsorbed onto sawdust or diatomaceous earth it becomes significantly less sensitive.
Today dynamite is manufactured by absorbing nitroglycerin in a mixture of sawdust, wood pulp, starch, and similar carbon-rich materi als. Calcium carbonate is added to neutralize the nitric acid that forms by spontaneous decomposition. Ethylene glycol dinitrate is added as an anti.- freeze and oxidizers are also typically added. The mixture is commonly
TERRORISM AND WMDs: AWARENESS AND RESPONSE![]()
formed into short sticks and wrapped in waxed paper; another popular configuration is as cast boosters (Figure 6.9).
Dynamite is primarily used in mining, quarrying, and construc tion activities. It is not used for military purposes due to its unstable nature, especially when subjected to freezing. Dynamite has been replaced for combat use by military dynamite, a mixture of TNT, RDX, binders, and antifreeze agents. Military dynamite has approximately 60% of the strength of nitroglycerin-based commercial dynamite (U.S. Army, 1990).
Dynamites vary widely in composition and are identified by manu facturer and trade name (Apache, Trojan, etc.), strengths (20%, 40%, 60%), application (ditching, quarrying), content of additives (gelatin, nitroglyc erin, ammonium nitrate), and form (bulk, slurries, water gels). Three forms of dynamite are in common use: straight dynamite, ammonia dynamite, and gelatin dynamite (Table 6.3). All are composed of the ingredients listed above; however, ammonia dynamite and straight dynamite contain ammonium nitrate and sodium nitrate, respectively, as oxidizers; gelatin dynamite contains 1% nitrocellulose that serves to thicken the nitroglyc erin and give the dynamite additional shattering power (Meyer, 2004).
Because of its enhanced stability over nitroglycerin, dynamite may be transported and used with less hazard of spontaneous detonation. Dynamite is so insensitive that a detonating cap is required for initiation.

Figure 6.9 Dynamite cast boosters.
EXPLOSIVE HAZARDS![]()
Table 6.3 Chemical and Physical Properties of Some Forms of Dynamite
| Straight Dynamite | Ammonia. Dynamite | Gelatin Dynamite | ||
| Density, g/mL | 1.3 | 0.8-1.2 | 1.3-1.6 | |
| Chemical composition | 20%-60% nitroglycerin | 12%-22% nitroglycerin | 20%-91% nitroglycerin | |
| 23%-60% sodium nitrate | 15%-57% sodium nitrate | 40%-60% sodium nitrate | ||
| 12%-50% ammonium nitrate | Gelatinized in 11itrocellulose | |||
| Detonation | ||||
| velocity, m/ s | 3600-6000 | 2700--4600 | 4000-7400 | |
| fps | 11,800-19,700 | 8900-15,000 | 13,000-24,000 | |
| Sensitivity | High | High | High |
Sources: Meyer, E., Chemistry of Hazardous Materials, Fourth Edition. Prentice Hall, Englewood Cliffs, NJ, 2004; U.S. Department of theArmy. Military Explosives. Technical Manual. TM 9-1300-214. Washington, DC, 1990.
Regardless, however, dynamite is considered to be sensitive to heat, shock, and friction and must be handled with extreme care. Aged dynamite can be quite hazardous. Old dynamite sticks that appear to be "sweat ing," with salt crystals forming on the outside of the package, dark stains appearing on the package cover, or liquid collecting at the base of a car tridge, indicate the presence of nitroglycerin that has leached from the dynamite. Such a scenario is extremely dangerous and highly susceptible to initiation.
Workers engaged in the production or use·of dynamite, or those touching or inhaling fumes from dynamite may become exposed to vapors of nitroglycerin and/or ethylene glycol dinitrate. Initial expos ure often results in an intense headache and in some cases dizziness, nausea, palpitations, and decrease in blood pressure. These initial symp toms indicate a shift in blood volume from the central to the peripheral circulatory system initiated by dilation of blood vessels (Globalsecurity. org, 2008).
The U.S. DOT regulates the transportation of dynamite as a division l.lD explosive. The proper shipping name is "Explosive, blasting, type A." Containers of dynamite are labeled EXPLOSIVE 1.lD, and transport vehi cles must be placarded EXPLOSIVES l.lD.
TERRORISM AND WMDs: AWARENESS AND RESPONSE
Ammonium Nitrate-Fuel Oil
In 1956 a new blasting agent was developed using a mixture of ammo nium nitrate, aluminum powder, and water. The safety and efficiency of this new agent was soon apparent and subsequent research resulted in the development of slurry explosives and eventually to dry explosive agents.
Ammonium nitrate, chemical formula NH4NO:i, is a simple salt and a common commercial fertilizer. Mixed with fuel oil and confined, this material can be transformed into a powerful high explosive. Ammonium nitrate-fuel oil (ANFO) has a broad range of detonation velocities, depend ing on the reference cited. An approximation for large quantities of blast ing agent is roughly half the detonation velocity of C-4, or about 13,000 fps (Globalsecurity.org, 2008). ANFO blasting agents are among the most common industrial explosives used in the United States. ANFO has
essentially replaced dynamite for bench blasting during surface mining and quarrying. The most widely used dry blasting agent is a mixture of ammonium nitrate prills and fuel oil.
Explosive grade ANFO is the least sensitive of all manufactured explosives. For safety reasons, the components are often stored separately in warehouses and mixed at the point of use. However, the mixed prod uct can also be purchased (Figure 6.10). ANFO is relatively safe and eas ily handled and can be poured into drill holes in the rock material to be blasted.

Figure 6.10 ANFO mixture. (US Bureau of Alcohol, Tobacco and Firearms.)
EXPLOSIVE HAZARDS![]()
Military Explosives
Military explosives are not readily available to civilians. However, thefts have occurred, resulting in their incorporation in improvised bombs. For responders, these explosives should be handled with the same safety pre cautions as those employed with commercial explosives.
RDX
RDX is a nitramine compound with the chemical formula 1,3,5-trinitro- 1,3,5-triazine. Its structure is a heterocycle and is shown in Figure 6.11. Several explanations exist for the term RDX, including Royal Demolition Explosive, Research Department Composition X and Research Department Explosive (Cocroft, 2000). It is more commonly known as cyclonite or hexogen.
RDX was used widely during World War II because petroleum was not required as a raw ingredient. Since World War II, RDX has become the second-most widely used high explosive in the military, exceeded only by TNT.
RDX is typically used in mixtures with other explosives, oils, or waxes. It is considered the most powerful and brisant of the military high explosives (see Box 6.4). RDX is second in strength to nitroglycerin among
Figure 6.11 Structure of RDX molecule.
;· ·,<_/ ::-.(->,.< .![]()
_., i \\'.
Brisance]-s/a 1;Il(:aSll.re of.the .speed wit .:which an explosive pro-·...... duces tts Ill Xi1;I!um. blastpresstJ.J:e. Tl:\is vah.J.e essentially describes
..•• the dest,ucti".' fragm nt<1.tim,effect of a Inat rial on its iinrn cl.}ate
; surroffildings. Also knuwn a shil.ttering · £feet, bris\l?ce· (frorr{Jhe .
. French briser,.0t o break") iSi?fpracticalilllportance indetrrrnining.
·.the effe6tivenessof anexplosionin.fragmeritfog structures,••
TERRORISM AND WMDs: AWARENESS AND RESPONSE
common explosive substances. When compressed to a density of 1.70 g/ cm3 it has a confined detonation velocity of about 27,000 fps (Table 6.1).
The higher the force and velocity of detonation, the greater the bri sance and shattering effect. Factors contributing to brisance include det onation rate and loading density (i.e., degree of compaction of explosives) and the heat of explosion and gas yield. High-brisant explosives are used for blasting hard rock and low-brisant explosives are used where a more pushing or heaving than shattering effect is needed.
RDX has been produced by several methods, but the most common manufacturing process in the United States is the continuous Bachmann process, which involves reacting hexamine with concentrated nitric acid, ammonium nitrate, glacial acetic acid, and acetic anhydride.
![]()
At room temperature RDX has a high degree of stability in storage; however, it becomes very sensitive when crystallized to temperatures below -4°C. RDX starts to decompose at about 170°C and melts at 204°C (Girard, 2007).
RDX is used alone or with other explosives, including PETN. It is widely used as a component of plastic explosives, detonators, high explo sives in artillery rounds, Claymore mines, and demolition kits. RDX can be mixed with plasticizers to make C-4 (a plastic explosive; see below). Semtex, another plastic explosive, combines RDX and PETN. RDX forms the base for the following common military explosives (Global Security, 2008):
Composition-A (RDX melted with wax)
Composition-B (RDX mixed with TNT)
Composition-C (RDX mixed with plasticizer)
HBX
H-6
Cyclotol
Civilian applications of RDX include use in fireworks, in demolition, and as a rodenticide. Combinations of RDX and HMX comprise the main ingredients in approximately 75 explosive products.
TNT
TNT (2,4,6 trinitrotoluene) is one of the most common bulk explosives in use today, both in military munitions and in civilian mining and
EXPLOSIVE HAZARDS![]()
quarrying activities. TNT was first used on a wide scale during World War I. TNT has the chemical formula C6Hi NO;J3CH3 and its structure, that of a nitroaromaiic compound, is shown in Figure 6.12.
The explosive yield of TNT is considered the standard of strength for comparison of explosives (see Box 6.5). TNT is classified as a second ary explosive because it is less susceptible to initiation and requires a primary explosive for ignition. TNT can be used as a booster for high explosive shells and bombs; it can also be mixed with other explosives such RDX and HMX and is a constituent of amatol, pentolite, tetrytol, torpex, tritonal, picratol, and others. TNT has been used under trade names such as Triton, Trotyl, Trilite, Trinol, and Tritolo (Global Security, 2008).
TNT is popular in the military and industry because of its insensitivity to shock and friction, which reduces the risk of accidental detonation.
TNT is synthesized in a three-step process (U.S. Army, 1990). Toluene is nitrated with a mixture of sulfuric and nitric acid to produce mono nitrotoluene (MNT). The MNT is then nitrated to dinitrotoluene (DNT). Lastly, DNT is nitrated to trinitrotoluene or TNT. The acids used in the manufacturing process are reused.
Figure 6.12 Structure of TNT, trinitrotoluene.
BOX6.5 RELATIVE EFFECTIVENESSFA.CTOR
FOR EXPLOSIVES
. The rela;i e effectiv ess factor. (cl!RE factllr) is measure ofthe power of an explosive for military demolitions (Table 6.4). The RE factor compares an explosive's effectiveness relative to TNT. This is so engineers can substitute one explosive for another when they are calculating blasting equations that are designed for TNT. For. exam- ····
ple, if a steel cutting charge requires 1 kg of TNT to work, it would take 0.6 .kg of PETN or 1.25 kg ofANFO to cause the same effect (Cooper/1996). · ·
TERRORISM AND WMDs: AWARENESS AND RESPONSE
Table 6.4 Examples of R.E. Factors for Explosives
Detonation
Density Velocity R.E.
Explosive g/cm> mis fps Factor
TNT 1.65 6900 22,640 1.00
Amato!, 80% TNT + 20% AN 1.55 6570 21,560 1.17
Ammonium nitrate 1.12 5270 17,290 0.42
ANFO, 94.3% AN+ 5.7% fuel oil 0.84 5270 17,290 0.8
C-4,91%RDX 1.74 8040 26,380 1.34
Composition-B, 63% RDX + 36% TNT 1.75 8000 26,250 1.35
Gunpowder, 75% KN 0 3 + 15% C+10 %S 1.7 Varies Varies 0.55
HMX 1.91 9100 29,860 1.70
Nitroglycerin 1.6 7700 25,260 1.50
Octanitrocubane 2.0 10,100 33,140 2.7
PETN 1.77 8400 27,560 1.66
ROX 1.82 8750 28,710 1.60
Semtex, 94.3% PETN + 5.7% ROX 1.78 8420 27,630 1.66
Tetryl 1.73 7570 24,840 1.25
Tetrytol, 70% tetryl + 30% TNT 1.71 7370 24,180 1.20
Sources: Cooper, P., Explosives Engineering. Wiley-VCH Publishing, New York, 1996.; U.S. Department of the Army. Field Manual 5-25 Explosives nnd Demolitions. Washington DC, 1967.
Upon detonation, TNT decomposes as follows:
2C7H5N3O6 -+ 3N2 + SH2O + 7CO + 7C (6.4)
TNT is poisonous to biota. Skin contact causes local irritation, caus ing the skin to turn a bright yellow-orange color. Persons exposed to TNT over prolonged periods (e.g., workers who manµ.factured TNT during the 1950s) experience increased risk of ailments including aplastic anemia and liver damage (e.g., toxic hepatitis) (ATSDR, 1996). Improved industrial safety practices have resulted in a significant decline in serious health problems related to TNT exposure.
As mentioned earlier, TNT is classified as a nitroaromatic compound (Figure 6.12). The majority of nitroaromatics occurring in the biosphere are industrial chemicals, including explosives, dyes, pesticides, and solvents.
-.- ... ,,
256
EXPLOSIVE HAZARDS
Nitroaromatics are recalcitrant xenobiotic compounds, that is, they are resistant to natural decomposition processes and tend to persist in the biosphere. In addition, nitroaromatics pose toxic and mutagenic hazards to humans, fish, and other biota.
PETN
Pentaerythritol tetra.nitrate (PETN) is one of the most powerful high explo sives known, with a confined detonation velocity of more than 25,000 fps and a relative effectiveness factor of 1.66. PETN is primarily used in booster and bursting charges of small caliber ammunition, in charges of detonators in some land mines and shells, and as the charge within deto
nating cord (Global Security, 2008). The formula of PETN is C5H8N4O12 and its structure is shown in Figure 6.13.
Manufacture of PETN involves nitration of pentaerythritol with a mixture of concentrated nitric and sulfuric acid. An alternative method of nitration uses concentrated nitric acid alone:
![]()
PETN was first synthesized in 1894 by the above method. This formula tion was subsequently used by the German army in World War I. PETN is also one of the ingredients in Semtex plastic explosive. Richard Reid (Abdel Rahim) attempted to use PETN to destroy a 767 jetliner traveling from Paris to Miami in 2001.
PETN also has medical applications; it is used as a vasodilator, similar to nitroglycerin. A treatment for heart disease, Lentonitrat, is nearly pure PETN (Russek, 1966).
Figure 6.13 Structure of PETN.
257 TERRORISM AND WMDs: AWARENESS AND RESPONSE
USE FORMS OF EXPLOSIVES
Pure explosive compounds may be used alone as liquids, powders, or solid casts; however, the majority of explosives require modification to their mechanical properties. To alter mechanical as well as other prop erties, pure explosives are blended with other explosives and with inert materials. The mixtures can then be manipulated to form specific explo sive products. The chief product forms include
Plastic
Cast
Sheet
Emulsion
Slurries
Detonating cords
Blasting caps
Binary mixtures
Plastic Explosives
In the context of explosives, the term "plastic" indicates a malleable or moldable material, similar to properties of modeling clay (Figure 6.14). Explosives are mixed with a plastic binder and a plasticizer to keep the material from hardening. Several plastic explosives have been popular among military engineers and for commercial (particularly industrial) uses. The most common commercial use of plastic explosives is for hard ening high manganese-percentage steel (PA&E, 2006). For the emergency responder, it should be noted that the ability of plastic explosives to
(a)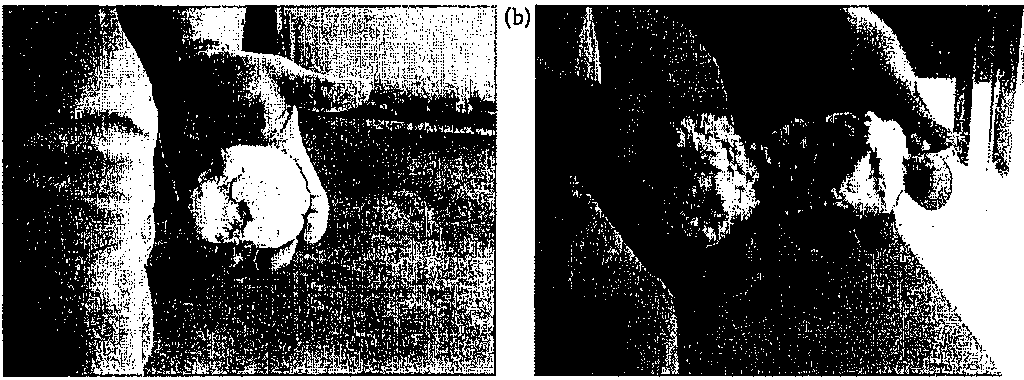
Figure 6.14 Sample of C-4, a plastic explosive.
EXPLOSIVE HAZARDS
conform to numerous shapes, while maintaining their destructive capa bilities, make them ideal weapons for IEDs for terrorists.
Common plastic explosives include C-4, which is quite popular with the U.S. military, and Semtex. Other popular plastic explosives and their country of origin are shown in Table 6.5.
C-4
C-4 ("Composition 4") is a malleable plastic explosive primarily used as a demolition charge, employed to cut through steel (Figure 6.14). C-4 is a simple mixture composed of explosives, plasticizer, and binder. As is common with many plastic explosives, the explosive material in C-4 is RDX, comprising about 90% of the C-4 by weight. This formulation also contains about 5% 2-ethylhexyl sebacate (the plasticizer), 2% polyisobuty lene (the binder), and 2% motor oil (Liberty, 2008).
C-4 is manufactured by combining RDX slurry with binder dissolved in a solvent. The solvent is evaporated and the mixture is dried and filtered.
Table 6.5 Various Plastic Explosives and Country of Origin Common Name Country
C-3, C-4
CHEMEX (C-4), TVAREX 4A
C-4 (Composition C-4), Detasheet, Primasheet, plastic-bonded explosives
KNAUERIT PENO
PE4, PLASTRITE (FORMEX P 1)
PLASTITE produced by SSE PE4,DEMEX
PWM, NITROLIT
PW-SA Plastic Explosive PP-01 (C-4)
Semtex Sprangdeg m/ 46
Sprengkiirper DM12 (formbar) T-4 Plastico
Greece
Slovakia
United States Austria Finland France Switzerland
United Kingdom Poland
Russia Yugoslavia Czech Republic Sweden Germany
Italy
Source: Thurman, J.T., Practical Bomb Scene Investigation. CRC Press, Boca Raton, FL, 2006; Explosia.cz. (http://www.explosia. cz/ en/?show=semtex.)
TERRORISM AND WMDs: AWARENESS AND RESPONSE
The final product is an off-white solid that feels similar to modeling clay (Figure 6.14). It is manufactured in block form.
detonates with a blast wave of approx. 8040 m/s (26,400 fps). For successful detonation C-4 requires the heat generated by the shock of a special military blasting cap. Commercial blasting caps are typically not powerful enough to detonate C-4.
When molding C-4, cracks may from in the explosive. These cracks will prevent the complete detonation of the charge and may result in scat tering of a portion of unused explosive. Such a phenomenon is termed a low-order explosion, that is, an incomplete detonation. In such a situa tion, hazardous material will persist on site, which may be susceptible to accidental detonation.
Military troops have become ill from exposure to C-4, which contains 90% RDX. Field exposures have been the result of soldiers either chew ing C-4 as an intoxicant or using it as a fuel for cooking. Scores of U.S. soldiers experienced convulsions due to RDX ingestion during the Vietnam War.
Semtex
Semtex is another popular malleable explosive, with characteristics and uses similar to those of C-4. Semtex was first manufactured by the Semtin East Bohemian Chemical Works in the Czech Republic. It is used in com mercial blasting, demolition, and in certain military applications. As with C-4, Semtex can be used to cut through thick steel.
Semtex uses high explosive (PETN mixed with RDX), combined with a binder of a synthetic styrene-butadiene rubber binder, resulting in a malleable product. Semtex is brick-orange in color, while C-4 is off-white. Semtex has the benefit of being usable over a greater temperature range than other plastic explosives. The two common varieties of Semtex are"k' (for blasting) and "H" (for hardening). Details regarding composition are presented in Table 6.6.
Cast ExplosivesCast explosives typically are shaped within cardboard, plastic, or metal packaging. An example of a cast explosive is TNT (see Figure 6.9). Cast explosives are relatively insensitive and normally consist of multiple com ponents. A responder should never assume that cast explosives only exist with their exterior packaging intact-such explosives do not require pack aging. Unexploded artillery and mortar projectiles have been stolen from
EXPLOSIVE HAZARDS
Table 6.6 Properties of Semtex A and H Forms
Semtex A SemtexH
PETN
RDX
Dye Antioxidant Plasticizer
Binder
76%-94%
4.6%-5.7%
Sudan IV (reddish brown) N-phenyl-2-naphthylamine di-n-octyl phthalate,
tri-n-butyl citrate
Styrene-butadiene rubber
41%-50%
41%-50.2%
Sudan I (red-orange)
N-phenyl-2-naphthylamine
di-n-octyl phthalate, lri-n-butyl citrate
Styrene-butadiene rubber
Sources: Liberty. 2008. http://www.libertylib.com/semtex.shtml#semtex-ingre dients; Hobbs, J.R.Analysis of Semtex explosives. In Advances in Analysis and Detection of Explosives, Kluwer Academic Publishers, the Netherlands, 1993; Yinon, J., and Zitrin, S. Modern Methods and Applications in Analysis of Explosives. Wiley and Sons, New York, 1993; Answers.com. http:// v.,ww.answers.com/topic/semtex.
(a)
Figure 6.15 Sheet explosive.
ranges at military bases, cut open, and the cast explosives removed and employed in IEDs.
Sheet ExplosivesAlso known as plastic-bonded explosives (PBX), Detasheet or Flex-x, sheet explosives consist of a mixture of PETN or RDX, and a polymer such as polystyrene. Due to its flexibility its major use is as a cutting charge for irregular surfaces.
In sheet explosive the fine explosive particles are embedded in a rub bery binder. The combination of explosive and polymer is extruded into
TERRORISM AND WMDs: AWARENESS AND RESPONSE
rolls, sheets, ribbons, and cord. The sheets measure between 1 mm and 8 mm thick and are available in rolls with inner layers of waxed paper preventing adhesion of the explosives (Figure 6.15). Sheet explosives for military use are manufactured in olive drab color; however, commercial sheet explosives may be found in any color.
Sheet explosives are extremely insensitive to shock-they require a blasting cap for initiation. Sheet explosives rate among the most powerful and versatile of explosives. They are appealing to terrorists because they can be hidden in envelopes, briefcases, or even sewn into the clothing of a suicide bomber.
Emulsion ExplosivesAn emulsion is composed of a mixture of two immiscible liquids, that is, those which do not normally mix with one another. One liquid is typically oil-based, while the other is water-based. When the appropriate emulsi fier is included in the mixture, the two phases blend well together.
Emulsion explosives are prepared as water-in-oil emulsions. One phase is composed of an oxidizer salt solution (e.g., ammonium nitrate) suspended as microscopic-sized droplets surrounded by a continuous fuel phase. An emulsifying agent stabilizes the mixture. Since each microcell of the oxidizer is coated with an oily exterior, the emulsion has excellent water resistance. A bulking agent such as ultrafine air bubbles may be dis persed throughout the emulsion matrix, which modifies the sensitivity of the explosive (Zukovich, 2008). Many fuels are incorporated in emulsion explosives, for example, water-soluble organics such as glycol and alco hols. Some emulsion explosives have a high content of aluminum powder that increases overall explosive force and imparts a shiny metallic color when the contents are exposed.
Emulsion explosives were originally manufactured for commercial use but now have military applications as well. They can be identified by their packaging in flexible plastic tubes (Figure 6.16). The exterior wrap ping can be any color.
Slurry ExplosivesSlurries, sometimes called water gels, contain a mixture of oily and water based components in proportions opposite those of emulsions. Whereas an emulsion contains aqueous materials (ammonium nitrate in water) dis persed in a fuel matrix, slurries consist of tiny droplets of fuel dispersed
EXPLOSIVE HAZARDS
Figure 6.16 Emulsion explosive.
within aqueous oxidizers. Explosive slurries typically contain ammonium nitrate in solution. Depending on the remainder of the ingredients, slur ries are classified as either blasting agents or explosives. Slurry blasting agents contain nonexplosive sensitizers or fuels such as carbon, sulfur, or aluminum, and are extremely insensitive to initiation. Slurry explo sives, on the other hand, contain blasting cap-sensitive ingredients such as PETN or TNT.
The sensitivity and the detonation properties of the explosive mix ture are adjusted by the addition of ultrafine air bubbles. Such air spaces are required for the stable propagation of the detonation wave through the mixture. Slurries are thickened with gelatins, guar gums, and water soluble polymerizable plastics which also impart water resistance (Cooper and Kurowski, 1996).
Blasting Agents
Blasting agents are insensitive explosive mixtures usually made with ammonium nitrate (Figure 6.17) as the oxidizer, and a petroleum-based fuel. Such agents may contain additional substances such as powdered aluminum or ferrosilicon to increase density. Blasting agents may be dry or in slurry forms. Because of their insensitivity, blasting agents are often detonated by a primer of high explosive.
263TERRORISM AND WMDs: AWARENESS AND RESPONSE
Figure 6.17 Ammonium nitrate prills as a blasting agent.
Advantages of using insensitive dry blasting agents are their safety, ease of loading, and low cost. In the free-flowing form they have a great advantage over cast explosives because they completely fill a mining bore hole. Such close bonding with the walls ensures efficient use of explosive energy.
Binary ExplosivesIn binary explosives, two nonexplosive components are mixed immedi ately before use to create a blasting cap-sensitive high explosive. Astrolite is formed when ammonium nitrate is mixed with anhydrous hydrazine. This produces a clear liquid explosive called Astrolite G. Astrolite G gen erates a very high detonation velocity, almost twice as powerful as TNT (Global Security, 2008). When fine aluminum powder is added to the slurry, another explosive, Astrolite A-1-5, is formed.
Blasting CapsA blasting cap is a small explosive device used to detonate a larger, more powerful explosive (Figure 6.18). The blasting cap contains a highly sensi tive primary explosive that provides the initial activation energy to trig ger a detonation in a more stable explosive. Explosives commonly used in caps include lead azide, lead styphnate, mercury fulminate, sodium azide, and tetryl (Cooper, 1996).
264
EXPLOSIVE HAZARDS

Figure 6.18 Blasting cap.
There are two major classifications of detonators-electric and nonelec tric. Both contain very sensitive explosives and are highly susceptible to accidental insult. Electric blasting caps consist of a small metal tube closed at one end that contains the charge of sensitive primary high explosive. The cap typically contains an ignition charge, an intermediate charge, and a base charge. The firing element consists of two plastic insulated lead or leg wires, an insulated plug holding the leg wires in place, and a small bridge wire connecting the leg wires inside the ignition charge (Figure 6.19).
The cap is initiated using a source of electricity such as a battery. Upon application of electric current, the bridge wire heats to incandes cence and initiates the ignition charge. The resulting heat and flame sets off the intermediate charge, which sets off the base charge, which in turn detonates the main charge.
Nonelectric blasting caps are attached to one end of a safety fuse. The other end of the fuse can be ignited with a flame to initiate the explosive train. The ignition charge is designed to amplify the flame from the fuse. This flame front ignites the intermediate charge, which in turn detonates the base charge.
Detonating CordDetonating cord ("Det cord") is a thin, flexible cord packed with high explosive in its core. It is used in a firing train to initiate commercial high
265 -- ·· ',,,·_·.-:,,
TERRORISM AND WMDs: AWARENESS AND RESPONSE
Figure 6.19 Schematic diagram of an electric blasting cap.
explosives. Detonating cord is commonly used in mining, drilling, and demolition work. The core typically occurs as a white pressed form. The cord varies in thickness and the exterior may be a variety of colors (Figure 6.20). Detonating cord is actually a high-speed fuse, usually composed of PETN, which explodes rather than deflagrates. It is a relatively insensitive secondary explosive and requires a blasting cap toinitiate.The PETN within detonating cord explodes at 6300 m/s (21,000 fps). Because of this high speed of detonation, det cord can set off multiple charges simultaneously.
(a)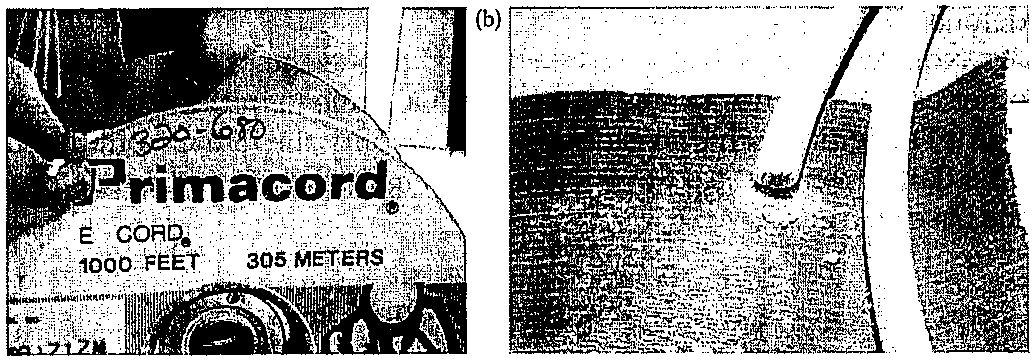
Figure 6.20 Detonating cord.
EXPLOSIVE HAZARDS![]()
Detonation cord will initiate most commercial high explosives (e.g., dyna mite) but will not initiate less sensitive blasting agents like ANFO.
Nonel Shock Tube
Nonel is a shock tube designed to initiate explosions, typically for demoli tion of buildings and in rock blasting in mines and quarries. As with det cord, shock tube firing systems employ a small plastic tube to deliver the firing impulse to the detonator. The hollow core is coated with a dusting of high explosive, typically PETN and fine aluminum. Shock tube operates by striking a plunger-like device such as a percussion-type fuse lighter or shock-firing device that causes the coated plastic tube to transmit a det onating wave. The reaction travels at approximately 2000 m/s (6500 fps) along the length of the tubing. At the end of the shock tube is a nonelectric blasting cap.
It is estimated that one pound of high explosive can be used for
approximately 70,000 feet of tubing.
Safety Fuses
Safety fuses provide a time delay to detonate the main explosive charge from a safe distance. A hobby fuse is composed of a low explosive, typi cally pressed black powder and potassium nitrate (Figure 6.21).
Figure 6.21 Hobby fuse.
TERRORISM AND WMDs: AWARENESS AND RESPONSE
The fuse is ignited at one end and burns at a controlled rate (allowing the user to reach a safe area) to the other end where a nonelectric blasting cap has been installed. The fuse imparts a flame to the blasting cap and initiates the explosive within. A safety fuse can also be used without a blasting cap to initiate a low explosive device containing black or smoke less powder as the main charge.
Safety fuses a.re available in many sizes and exterior colors, similar to detonating cord. Safety fuses can be identified, however, by their central core of black powder. The exterior is covered to protect against abrasion and water penetration.
EFFECTS OF AN EXPLOSION
An explosion undergoes three chief phases:
Blast
Fragmentation
Thermal
The severity of an explosion is a function of the type of explosive (e.g., detonation velocity, degree of brisance), the amount of explosive material involved, and conditions of the explosion (i.e., whether material was con fined; proximity to structures, etc.).
Blast Phase
The first phase of an explosion involves the creation of the characteristic blast (shock) wave immediately following detonation. Shock waves can result in widespread damage to surroundings, including structures and populations.
In the blast phase, the detonation generates gases under high pres sure (up to several thousand psi) and high temperatures (approximately 3000°C-4000°C). The hot gas expands, forcing out the volume it occupies. A layer of compressed air (the blast wave), containing most of the energy released by the explosion, consequently forms in front of this gas volume. The pressure of the blast wave instantaneously increases to a value well above the ambient atmospheric pressure. This overpressure can reach as high as hundreds of tons per square inch. The overpressure is sufficient to destroy structures and cause severe injuries and death.
The blast wave travels outward like ripples created by a stone dropped into a pond.Theinitial blast of outward pressure is often called the positive
EXPLOSIVE HAZARDS
phase of the blast. The side-on overpressure rapidly decays as the shock wave expands outward from the explosion source. When pressure from the positive phase extends outward a partial vacuum is created at ground zero (i.e., the center of the blast). This negative pressure is short-lived and is quickly filled by outside pressure after the blast subsides-air is rapidly sucked back into ground zero. This effect is the so-called negative phase of the explosion, and the "refilling" effect is termed implosion. Implosion provides for the rapid equalizing of the surrounding air pressure. The negative phase is accompanied by high winds that carry debris from long distances back into the original blast area. Implosion may last three times longer than the actual explosion (Figure 6.22).
2
 4
4
![]() 5
5
Figure 6.22 Time-series showing effects of a blast. (1) pre-detonation; (2) posi tive pressure from the blast; (3) stabilization (extremely brief); (4) negative pres sure; and (5) final stabilization.
TERRORISM AND WMDs: AWARENESS AND RESPONSE
Fragmentation PhaseThe second phase of an explosion is the fragmentation phase. In this phase the container of the exploding material is destroyed. Nearby objects are shattered to pieces and thrown outward in all directions. Many injur ies received from an explosion are often fragmentation wounds.
Thermal Effect PhaseThe final phase of an explosion is the thermal effects phase. This involves the release of heat generated from the blast. The rate of heat release and the temperature depend on the explosive material involved, the distance from the blast, and the speed of the explosion. Thermal effects can result in severe skin burns, death, and damage to materials such as metals, glass, and polymers.
EXPLOSIVES AND TERRORISM: IMPROVISED EXPLOSIVE DEVICESAn improvised explosive device (IED) is a term used to describe a home made bomb; it is a device intended for destructive use in nonmilitary applications. Many IEDS are made from simple, readily available mate rials such as steel or PVC pipes, standard electrical wiring, home-made switches, and consumer batteries (Figure 6.23). The explosive charge may contain smokeless powder or ANFO, or a more sensitive ingredient. Some IEDs are derived from military explosives attached to a detonating mech anism (Figure 6.24). IEDs are triggered by various methods, including remote control, infrared or magnetic triggers, pressure-sensitive bars, or trip wires.
IEDs are extremely diverse in design and may contain many types
of initiators, detonators, penetrators, and explosive loads. Improvised devices generally consist of four components: a power source, an initiator, the explosive charge, and a switch. Shrapnel may be added to the bomb housing.
Power SourcesMost IEDs contain an electric initiator and, therefore, require an electric power source such as a battery. Most commercially available batteries will
EXPLOSIVE HAZARDS
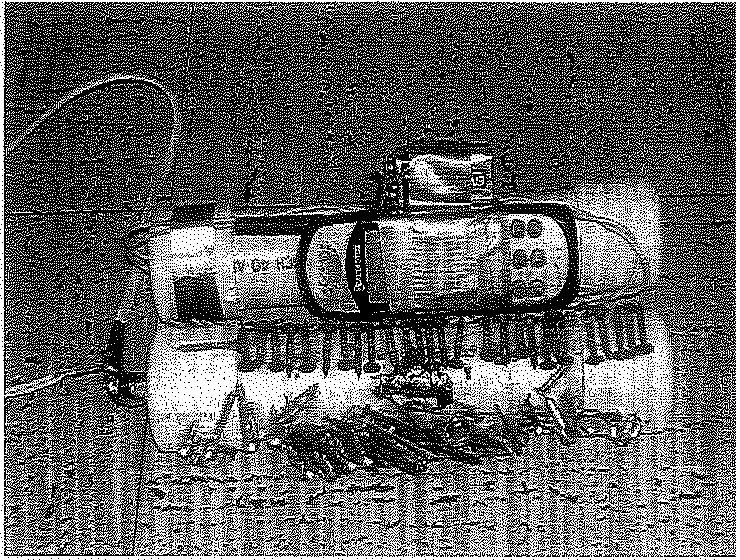
Figure 6.23 Improvised explosive device (IED).

Figure 6.24 Various IEDs recovered from an Iraqi bomb-making factory. These IEDs used U.S. munitions as the primary charge. (http://www.defenselink.mil/ news/Nov2.)
allow an initiator to function. Batteries can be cut and shaped to make detection more difficult.
InitiatorsThese consist of blasting caps or flame-producing components like fuse igniters. Improvised initiators can be easily constructed. Examples include
TERRORISM AND WMDs: AWARENESS AND RESPONSE
a modified flash bulb or a hobby fuse. Initiator chemical constituents can also be improvised; an example may include triacetone triperoxide (TATP).
ChargeExplosives take many forms in IEDs. Some are manufactured using old munitions, while others contain commercial ingredients (e.g., dynamite). Still others may contain simple ingredients purchased from a local hard ware store.
SwitchesSwitches are used as either an arming switch or a fuse and can be simple or complex in design. More than one switch can be included to create redun dancy in the system. The arming switch is a safety for the IED and works by disarming (i.e., electrically disengaging) the fusing switch. When the arming switch is engaged the fusing switch becomes functional. Designs for switches are essentially unlimited so any action by its intended target or a first responder could result in detonation. Switches designed specifi cally for IEDs can appear quite innocent-looking, completely fitting into the local surroundings.
Fragmentation and ShrapnelUpon detonation of an IED, the bomb housing and other attachments can cause injury and death. Fragmentation may consist of the original casing or the electronics within the IED. In contrast, shrapnel is intentionally added to the device to inflict maximum casualties. Examples of shrap nel include nails, BBs, ball bearings, steel washers, and children's marbles (Figure 6.25).
RECENT USE OF IMPROVISED EXPLOSIVE DEVICES
Afghanistan and Iraq
Since 2001, IEDs have been responsible for more than 60% of all American combat casualties in Iraq and 50% of combat casualties in Afghanistan, including both killed and wounded (U.S. DOD, 2007). Vehicle-borne IEDs are used to strike police stations, markets, and mosques, killing local
EXPLOSIVE HAZARDS
Figure 6.25 Improvised explosive device to which assorted shrapnel have been attached.
citizens as well as U.S. troops. Roadside IEDs have targeted U.S. military vehicles and caused immense damage and casualties.
The U.S. military recorded 8159 IED incidents in Afghanistan in 2009, compared with 3867 in 2008 and 2677 the year before. In contrast, the number of IED attacks in Iraq has plummeted, mirroring the overall decrease in violence in that country. At their peak, in 2007, Iraqi insurgents employed 23,000 IEDs. By 2009, that number fell to about 3000, according to U.S. military figures (Figure 6.26) (JIEDDO, 2009; Whitlock, 2010).
A large proportion of the munitions for constructing IEDs in Iraq may have originated from large Iraqi military ordnance deposits looted by insurgents (Glanz and Dwyer, 2004). Department of Defense officials have charged that Iran may be supplying new IED technology to insurgents in Iraq. In Afghanistan, the IED munitions supply is supported by funds from an expanding opium trade (Bergen, 2006). There is concern that IEDs might eventually be used by other insurgents and terrorists worldwide (Sadler, 2005).
In Iraq, small, highly skilled IED cells often hire themselves out to other insurgent groups, such as al-Qaeda in Iraq or the Sunni group Ansaar al Sunna. They advertise their expertise on the Internet, and may be con tracted on a per-job basis but otherwise remain autonomous (Times Wire, 2006). A typical IED terrorist cell may consist of six to eight people including
TERRORISM AND WMDs: AWARENESS AND RESPONSE
2000
W-Iraq
March '07
2612
2500 Afghanistan
'
1500
1000
2004 2005 2006 2007
Ii
August '09
1067
2008 2009 '10
Figure 6,26 Trends in IED attacks on Coalition Forces in Iraq and Afghanistan. (https://www.jieddo.dod.mil/content/docs/20100804_FULL_2009%20Annual%20 Report_Unclassified_vl.pdf.)
a financier, bomb maker, emplacer, triggerman, spotter, and often a camera man. Videos of exploding U.S. vehicles and dead Americans are distributed via the Internet to attract new supporters (Wilson, 2007).
U.S. forces are continually developing countermeasures for the devices, for example, by relying on intelligence sources and by disrupting portions of the radio spectrum that insurgents use to trigger IEDs. New technologies being evaluated include electronic jammers and predetona tors, radars, x-ray equipment, robotic explosive ordnance disposal equip ment, physical security equipment, and armor for vehicles and personnel (Lieberman, 2005). However, insurgents quickly adapt to countermea sures, and new, more sophisticated IEDs are increasingly being used in both Iraq and Afghanistan (Wilson, 2007).
Over the Atlantic, 2009Umar Farouk Abdulmutallab (Figure 6.27), a Muslim Nigerian citizen, attempted to detonate an improvised device hidden in his underwear while on board Northwest Airlines Flight 253en route from Amsterdam to Detroit, Michigan, on December 25, 2009. After spending about 20 minutes in the airplane restroom Abdulmutallab returned to his seat and covered himself with a blanket. Soon afterward passengers nearby heard popping
274 EXPLOSIVE HAZARDS![]()
Figure 6.27 Umar Farouk Abdulmutallab, arrested for allegedly attempting to bomb a Northwest Airlines flight using an !ED worn in his clothing. (http:// en.wikipedia.org/wiki/File: UmarFarouk.jpg.)
noises and smelled a foul odor. Some saw fire on Abdulmutallab's clothing and the wall of the plane. Fellow passengers jumped on Abdulmutallab and restrained him as flames were doused with fire extinguishers.
The device consisted of a six-inch (15-cm) packet sewn into his under wear containing both pentaerythritol (PETN) along with a TATP (Krolicki and Pelofsky, 2009). After being taken into custody, Abdulmutallab informed authorities that his actions were directed by al-Qaeda, and that he had received training and obtained the materials for the device while in Yemen. Al-Qaeda in the Arabian Peninsula, the organization's affiliate in Yemen, claimed responsibility for the attack, describing it as revenge for the U.S. role in a Yemeni military offensive against al-Qaeda in that country (Spiegel and Solomon, 2009).
Abdulmutallab was subsequently charged on six criminal counts, including attempted useof a WMD and attempted murder of 289 people. As of this writing he is in U.S. custody awaiting further legal proceedings.
London, U.K.On July 7, 2005, a series of coordinated suicide attacks occurred on London's public transport system during the morning rush hour. The bombings
TERRORISM AND WMDs: AWARENESS AND RESPONSE
were carried out by four British Muslim men, three of Pakistani and one of Jamaican descent.
At 8:50 a.m. three bombs exploded within 50 seconds of each other on three London Underground trains just outside Liverpool Street and Edgware Road stations, and on another traveling between King's Cross and Russell Square. A fourth bomb exploded at 9:47 a.m. on a double-decker bus in Tavistock Square. The explosions were apparently caused by home-made organic peroxide-based devices, packed into backpacks and detonated by the bombers themselves, all four of whom died. A total of 56 people, includ ing the four bombers, were killed and about 770 were injured.
The bombers were named as Mohammad Sidique Khan, age 30; Shehzad Tanweer (22); Germaine Lindsay (19); and Hasib Hussain (18). Two of the bombers made videotapes describing their reasons for becom ing "soldiers" for their cause. In a videotape aired by Al Jazeera on September 1, 2005 (Figure 6.28), Mohammad Sidique Khan described his motivation.
I and thousands like me are forsaking everything for what we believe. Our drive and motivation doesn't come from tangible commodities that this world has to offer. Our religion is Islam, obedience to the one true God and following the footsteps of the final prophet messenger. Your democratically elected governments continuously perpetuate atrocities against my people all over the world. And your support of them makes

Figure 6.28 Mohammad Sidique Khan, one of the four men who bombed the London transit system in July 7, 2005, in a video aired by Al Jazeera. (http://www. telegraph.co.uk/news/uknews/law-and-order/5351318/July-7-leader-Sidique Khan-encountered-security-services-10-times-before-bombings.html.)
EXPLOSIVE HAZARDS
you directly responsible, just as I am directly responsible for protecting and avenging my Muslim brothers and sisters. Until we feel security you will be our targets and until you stop the bombing, gassing, imprison ment and torture of my people we will not stop this fight. We are at war and I am a soldier. Now you too will taste the reality of this situation.
Forensic examiners had initially thought that military-grade plas- tic explosives were used in the attacks, and, as the blasts were thought to have occurred simultaneously, that synchronized timed detonators were employed. However, it was eventually determined that home-made organic peroxide-based devices were used (ISC, 2006). Each device was believed to contain 10 pounds or less of triacetonetriperoxide (TATP), and was small enough to fit inside a backpack.
Oklahoma City, USAOn April 19, 1995, a large vehicle bomb detonated on the north side of the Alfred P. Murrah Federal Building in Oklahoma City, OK (see Figure 1.1). A total of 168 perished in the blast; estimates of injuries varied from 460 to more than 800. The true number may never be known, as many treated themselves and did not seek medical help as they realized that medical facilities in the area were overwhelmed.
The Murrah Federal Building was constructed in 1977 of rebar-rein forced concrete. The structure had nine floors with basement and parking garages and included more than 300,000 square feet, providing office space for various federal agencies, a credit union and child day care center.
The detonation rocked every building in Oklahoma City. The explo sion destroyed the Murrah Building, the Water Resources Board, a res taurant and other structures in the surrounding area as well as breaking glass windows and doors for fourteen blocks. A total of 342 businesses were damaged and ten different structures were destroyed. The north face of the Murrah Building collapsed and numerous vehicles in the parking lot across 5th Street ignited into flames, sending smoke and an enormous concrete dust cloud over the area.
The blast was felt 50 miles away, causing security alarms throughout the city to activate. The Oklahoma Geological Survey Station, located 16 miles south of Oklahoma City, recorded a large surface wave at 9:02:13
followed by a second wave at 9:02:23 a.m.
Oklahoma City Bomb Squad Officers quickly assessed the damage as consistent with a large vehicle bomb and not that of an accidental explo sion. Since the bombing was of a federal building and federal employees
TERRORISM AND WMDs: AWARENESS AND RESPONSE
were killed, the Federal Bureau of Investigation (FBI) was designated the lead criminal investigative agency.
Approximately 460 tons of debris was sifted and examined for evi dence. All vehicles in the lot across the street were processed and the owners identified. As investigators completed assignments in the outer areas, they were moved into other teams working the inner perimeter.
The first key piece of evidence discovered was a large section of the vehicle used in the bombing, located 150 feet from the blast site. A rear axle and gear housing from a large vehicle was located in front of the Regency Towers Apartment Building and the axle contained a vehicle identification number (VIN). Investigators initiated a trace of the VIN and found it to be from a 1993 Ford F700 cargo van registered to Ryder Truck Rental and bearing Florida license NEE26R.
On the basis of evidence collected at the scene, interviews with wit nesses and evidence obtained through the arrest of Timothy McVeigh and execution of several search warrants, the device was determined to be a large vehicle bomb contained in a 1993 Ford F700 20 foot cargo van. The main explosive charge contained approximately 4800 pounds of ammo nium nitrate and nitromethane mixture packed in blue and white plastic barrels. The firing train of the device was a fuse igniter of approximately 4 feet of safety fuse with a nonelectric detonator. The explosives boost ers could not be identified and may have been a combination of explo sives. The main charge was located in the cargo box. It is probable that McVeigh parked the vehicle in the freight zone of the Murrah Building on 5th Street, ignited the safety fuse from the cab, exited, locked the vehicle, and walked away. The fuse was attached to the explosive train through a hole penetrating the cab to the cargo box. The fuse would have burned at approximately 42 seconds per foot or 2½ minutes total time.
The vehicle-borne IED detonated at a velocity of 8000 fps, creating a significant thermal effect that ignited vehicles nearby. A blast pressure wave shattered the glass face of the Murrah Building. Persons inside were thrown through the air, causing massive injuries from the pressure wave and shock front, as well as from shards of broken glass and flying debris. The blast wave entered the Murrah Building causing failure of the main transfer beam that provided support to the third through ninth floors. As this beam collapsed, all floors along the north wall collapsed in a pancake configuration to the street below. The crater was approximately 30 feet
wide and 8 feet deep.
! The blast wave penetrated but was also reflected off the nearby Journal
' Record Building across 5th Street. Vehicles in the area received extensive
EXPLOSIVE HAZARDS
damage from blast pressures that were traveling at more than 7000 miles per hour.
Nine buildings in a four-block area were destroyed by the blast. A tenth structure approximately a quarter-mile north of the Murrah site collapsed as a result of reflective pressure. Structural damage was noted as far away as five blocks from the blast seat. Fragments of the vehicle containing the explosives were recovered. up to eight blocks from the bomb crater.
Timothy McVeigh was arrested by an officer of the Oklahoma Highway Patrol at 10:22 a.m. on the day of the bombing about 75 miles north of Oklahoma City. Evidence recovered from McVeigh's clothing and vehicle assisted in the device reconstruction. Residue samples of the explosive chemical PEIN, commonly used in detonating cord, were found on McVeigh's knife and in his pockets. Residue samples of the explosive chemical EDGN, a chemical used as an antifreeze in dynamite, were recovered from earplugs removed from McVeigh's vehicle.
On June 2, 1997, McVeigh was found guilty on eleven counts of mur der and conspiracy and was sentenced to death. He was executed by lethal injection at a U.S. penitentiary in Terre Haute, Indiana. An accomplice in the bombing, Terry Nichols, was convicted in Federal Court for con spiracy to commit murder of federal law enforcement officers and man slaughter of the federal officers. He was given a life sentence. The State of Oklahoma tried Terry Nichols for the murder of 162 victims. Nichols was convicted at the state level and received a life sentence. A third accomplice, Michael Fortier, was convicted in Federal Court for lying to investigators and transporting stolen property over state lines. He was given a lenient sentence in return for his testimony against Terry Nichols and Timothy McVeigh. He is currently in federal prison serving a 23-year sentence.
Delivery of the IED
Delivery method of an IED is limited solely by the imagination of the per petrator. Cars, trucks, motorcycles, and bicycles have been equipped with explosives and set to explode by the driver or by remote control. A car bomb can carry thousands of pounds of explosives and may be loaded with shrapnel to increase its deadly effects. Characteristic features of car bombs or vehicle-borne IEDs (VBIEDs) are that they appear weighed-down, and in some cases their interiors appear to have been stripped down and rebuilt.
Boats laden with explosives can be used against ship targets and posi tions along a coast (e.g., piers, buildings, pipelines, etc.). Suicide bomb ers used a boat-borne IED to attack the USS Cole, which was making a
TERRORISM AND WMDs: AWARENESS AND RESPONSE
refueling stop in the Yemeni port of Aden in October 2000. Seventeen American sailors were killed and 39 were injured. U.S. and U.K. troops have also been killed by boat-borne IEDs in Iraq (BBC, 2006).
Numerous modes have been employed to deliver an IED to the desired location. In a 1920 attack at the New York Stock Exchange a horse-drawn cart was used to deliver explosives to the target site. During World War II, Mexican free-tailed bats were equipped with a small timed incendiary device and dropped from bombers over Japan. The bats would return to earth by parachute and roost during the day in attics and barns. Later on the timers would ignite the bombs. In 2003 eight people were killed when explosives strapped to a horse detonated in the market in the town of Chita, Colombia (BBC, 2003). Colombian authorities blamed the country's largest rebel group, the Revolutionary Armed Forces of Colombia (PARC), for the attack. Donkey bombs were a common tactic of the Sendero Luminoso (Shining Path) in Peru. Donkeys have been used for suicide attacks in Israel {Times, 1985) and in Afghanistan (Times, 2009).
Suicide bombing refers to an individual wearing explosives and det onating them to kill others, including themselves. The bomber conceals explosives on their person, in some cases in a specially-made vest (Figure 6.29) and a trigger to detonate the explosives. Some terrorists consider themselves to be "smart bombs" in that they can deliver their weapon to

Figure 6.29 Bomb vest.
EXPLOSIVE HAZARDS
the exact desired point, thus giving the greatest chance of achieving suc cess than any other method of attack. In addition, there is the psycholog ical impact of terrorists prepared to deliberately sacrifice themselves for their cause (Hunter, 2008). So-called bra bombs by women suicide bomb ers have been documented. Hiding the explosives in a bra instead of a vest allows for the women to reveal their midriffs when searched (Rediff, 2004). In 2007 a Tamil Tiger suicide bomber had explosives hidden in her bra. She blew herself up outside the office of a Tamil minister (Agence Frances Presse, 2007). The most notorious high-profile suicide attack was by Thenmuli Rajaratnam, who was wearing either a belt with explosives or a bra bomb for the assassination of Indian Prime Minister Rajiv Gandhi.
In recent years explosives have been hidden inside the body. In a 2009 attack in Jeddah, Saudi Arabia, Abdullah Hassan Tali Al-Asiri hid explo sives in his rectal cavity in an attempt to kill Prince Mohammed Bin Nayef, the head of theSaudi security service. Al-Asiri blew himself up in the attack but his target escaped with only minor injuries. By hiding the explosives in a body cavity he managed to pass security checks before entering the prince's office. It is believed the explosives were detonated electronically.
With the advent of full-body airport scanners, concern is growing over the possibility of suicide bombers having explosives surgically implanted in various parts of the body before boarding airliners. The British secret service agency MIS has uncovered evidence that al-Qaeda is planning a new stage in its terror campaign by inserting "surgical bombs" inside people. There is speculation that male bombers would have the explosive concealed near their appendix or in their buttocks, while females would have the explosive material placed inside their breasts in the same way as figure-enhancing implants (Leake, 2010).
QUESTIONS
Draw the firing train of an explosion. Include and label all components.
The size of an explosion and consequent blast damage will vary as a function of what factors?
Define: deflagration; detonation; xenobiotic; low-order explosion; immiscible.
List three examples of primary explosives. Why are these highly energetic materials not commonly used as the main charge of military ordnance?
TERRORISM AND WMDs: AWARENESS AND RESPONSE
For what other purposes (besides explosives) is ammonium nitrate commonly used?
What is the purpose of the "Relative Effectiveness Factor"? Discuss.
Nitroaromatic compounds pose what kinds of hazards to humans and other biota? Be specific.
49 Code of Federal Regulations. 2007. Transportation. Subpart C-definitions, classification and packaging for class. New Jersey State Police Homeland Security Branch. No date. New Jersey HazMat Emergency Response Course. 06085. Special Operations Section, Technical Service Bureau, Hazardous Materials Response Unit. 3,d Edition. Trenton, NJ. http://edocket.access. gpo.gov / cfr_2007 / octqtr/pdf/ 49cfr173.50.pdf (accessed November 2, 2010).
Agence Frances Presse. 2007. Tamil bra bomber targets Sri Lanka minister. November 27, 2007. http://afp.google.com/article/ALeqM5hypJWWD9
ww4hQudZ5 VySlFeKWmhA (accessed November 2, 2010).
Agency for Toxic Substances and Disease Registry (ATSDR). 1996. 2,4,6-Trinitro toluene. September 1996. http://permanent.access.gpo.gov/lps21/tfacts81. html (accessed November 2, 2010),
BBC.com. 2006. Iraq boat attack personnel named. BBC News. February 14, 2006. http://news.bbc.co.uk/2/hi/uk_news/6146844.stm (accessed November 2, 2010).
BBC.com. 2003. 'Horse bomb' hits Colombia town. http://news.bbc.eo.uk/2/hi/ americas/3098746.stm (accessed November 2, 2010).
Beveridge, A. (ed.). 1998. Forensic Investigation of Explosions. CRC Press. Boca Raton, FL.
Central Pacific, 2005. Nitroglycerine! Terrible explosion and loss of lives in San Francisco. Central Pacific Railroad Photographic History Museum. 1866 Newspaper article. http://cprr.org/Museum/Newspapers/Nitroglycerine. html (accessed November 2, 2010).
Cocroft, W.D. 2000. Dangerous Energy: The Archaeology of Gunpowder and Military Explosives Manufacture. Swindon: English Heritage. ISBN 1-85074-718-0.
Cooper, P. 1996. Explosives Engineering. Wiley-VCH Publishing, New York. Cooper, P., and S.R. Kurowski. 1996. Introduction to the Teclmologi; of Explosives.
Wiley-VCH Publishing, New York.
Defense Update. 2004. Sheet explosives-Revisited. http://www.defense-update. com/2004/04/sheet-explosives-revisited.html (accessed November 2, 2010).
Explosia. 2003. Brief history of plastic explosive Semtex®. http://www.explosia. cz/ en/?show=semtex
Fordham, S. 1980. High Explosives and Propellants. Pergamon, New York.
2



