All of the questions are in this file down there
MYP IB
Criteria A, B and C
Chemistry Periodic Table Trends
Name_____________________________________ Dated______
Draw a square off of a periodic table and describe what each number is within the square.
Choose your favorite element and write its symbol below. Then write down which IUPAC group on the periodic table it is in. Choose your least favor element as well and write down its group.
Write down the reactions for the following and predict the products:
Fluorine gas reacts with lithium metal
Potassium metal reacts with water
Chlorine gas is bubbled through a sodium bromide solution.
The Periodic Table we currently use is derived directly from that proposed in 1869 by Mendeleev who had noticed patterns in the physical and chemical properties of the elements he had studied.
The diagram below shows the first ionization energies of the first 18 elements of the Periodic Table.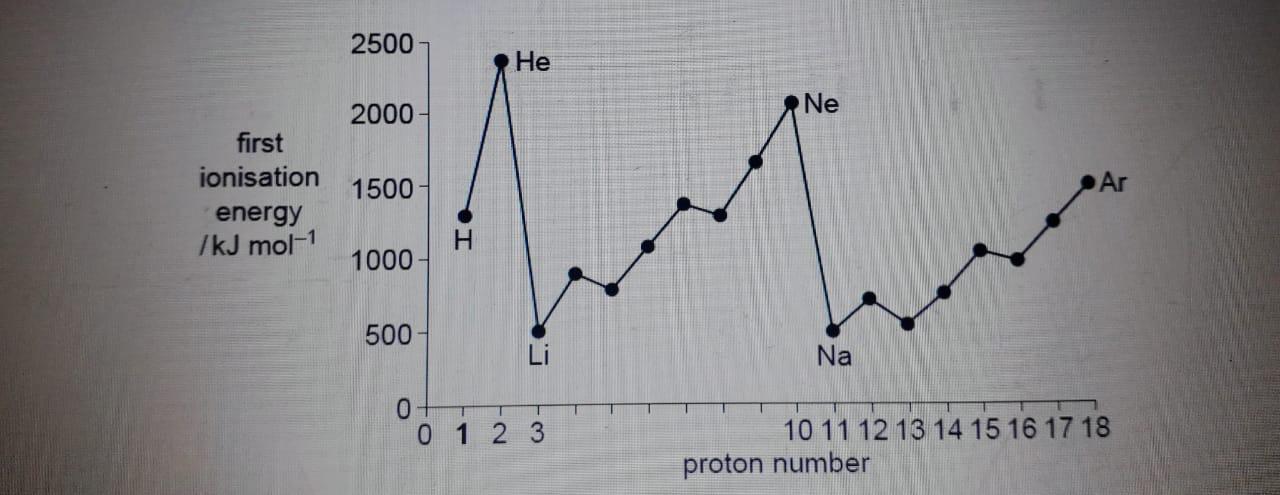
Give the equation, including state symbols, for the first ionization energy of carbon.
…………………...................................................................................................................................
(i) Explain why sodium has a lower first ionization energy than magnesium.
...............................................................................................................................................................
...............................................................................................................................................................
Explain why magnesium has a higher first ionization energy than aluminium.
...............................................................................................................................................................
...............................................................................................................................................................
Explain why helium, He, and neon, Ne, occupy the two highest positions on the diagram.
...............................................................................................................................................................
...............................................................................................................................................................
Explain why the first ionization energy of argon, Ar, is lower than that of neon, which is lower than that of helium.
...............................................................................................................................................................
...............................................................................................................................................................
...............................................................................................................................................................
(V) The first ionization energies of the elements Na to Ar show a variation. Some physical properties show similar variations.
The atomic radius of the elements decreases from Na to Cl. Give a brief explanation of this variation.
....................................................................................................................................................................................
....................................................................................................................................................................................
The cations formed by the elements Na to Al are smaller than the corresponding atoms.
Give a brief explanation of this change.
....................................................................................................................................................................................
....................................................................................................................................................................................
This question refers to the elements shown in the portion of the Periodic Table given below.
| He | ||||||||||||||||||||||||||||
| Li | Be | B | N | O | F | Ne | ||||||||||||||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||
| Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||||
From this table, identify in each case one element that has the property described. Give the symbol of the element in each case.
The element that has a molecule which contains exactly eight atoms.
....................................................................................................................................................................................
The element that forms the largest cation.
……………………………………………………………………............................................................
An element that floats on water and reacts with it.
....................................................................................................................................................................................
An element that reacts with water to give a solution that can behave as an oxidising agent.
....................................................................................................................................................................................
An element whose nitrate gives a brown gas on thermal decomposition.
....................................................................................................................................................................................
Elements in the same period of the Periodic Table show trends in physical and chemical properties. The grids on this page refer to the elements of the third period, Na to Cl.
On each of these grids, draw a clear sketch to show the variation of the stated property. Below each grid, briefly explain the variation you have described in your sketch.
For each explanation you should refer to the important factors that cause the differences in the property you are describing.
Explanation:
…………….......................................................................................................................
..........................................................................................................................................
..........................................................................................................................................
..........................................................................................................................................
(b)
Electronegativity of element
Na Mg Al Si P S Cl
Explanation:
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................
(C)
Electron Affinity of element
Na Mg Al Si P S Cl
Explanation:
...............................................................................................................................................
...............................................................................................................................................
...............................................................................................................................................



