Chemistry 131
Interdisciplinary Investigation (IDI)-Classroom
Research-based Interdisciplinary
Science Education
SARS-CoV-2 and a Global Pandemic: Investigating the Structure and Function of a Deadly Virus
The COVID-19 pandemic is caused by a virus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This virus was first identified in Wuhan, China in December 2019 and has now reached almost every corner of the globe. Many countries have been placed on various degrees of shelter-in-place orders as a strategy to slow the spread of the virus and hopefully prevent a large number of people from becoming ill at the same time.
In this investigation, you will explore the structure of this virus, learn about how a virus enters our cells and progresses through its life cycle, discover how soap is so powerful in disrupting the structure of this virus, and ultimately think about what therapeutics might be effective and treating or preventing this illness.
As part of this project, you will also be asked to participate in an “interdisciplinary thinking” challenge in order to better prepare for your future as a deep thinker and scientist. To complete this IDI , you will progress through the following parts of this project:
Preparation Activity (See Canvas)
Structure and Function of Soap & Connections to Biology
Structure and Function of Soap and Viruses
A. Viruses (Just in time Biology)
Are viruses made of cells? Do biologists consider them to be alive?
What are the basic components of a virus?
What are the five major steps of the viral life cycle?
What types of molecules make up the envelope of the SARS-CoV-2 virus?
What is the name of the receptor on our cells that binds with the spike protein of SARS CoV-2?
B. Identifying the functional groups that are significant in the structure and function of a soap.
4. Circle the ester or carboxylic acid functional group on each of the following molecules and name each functional group you circle.
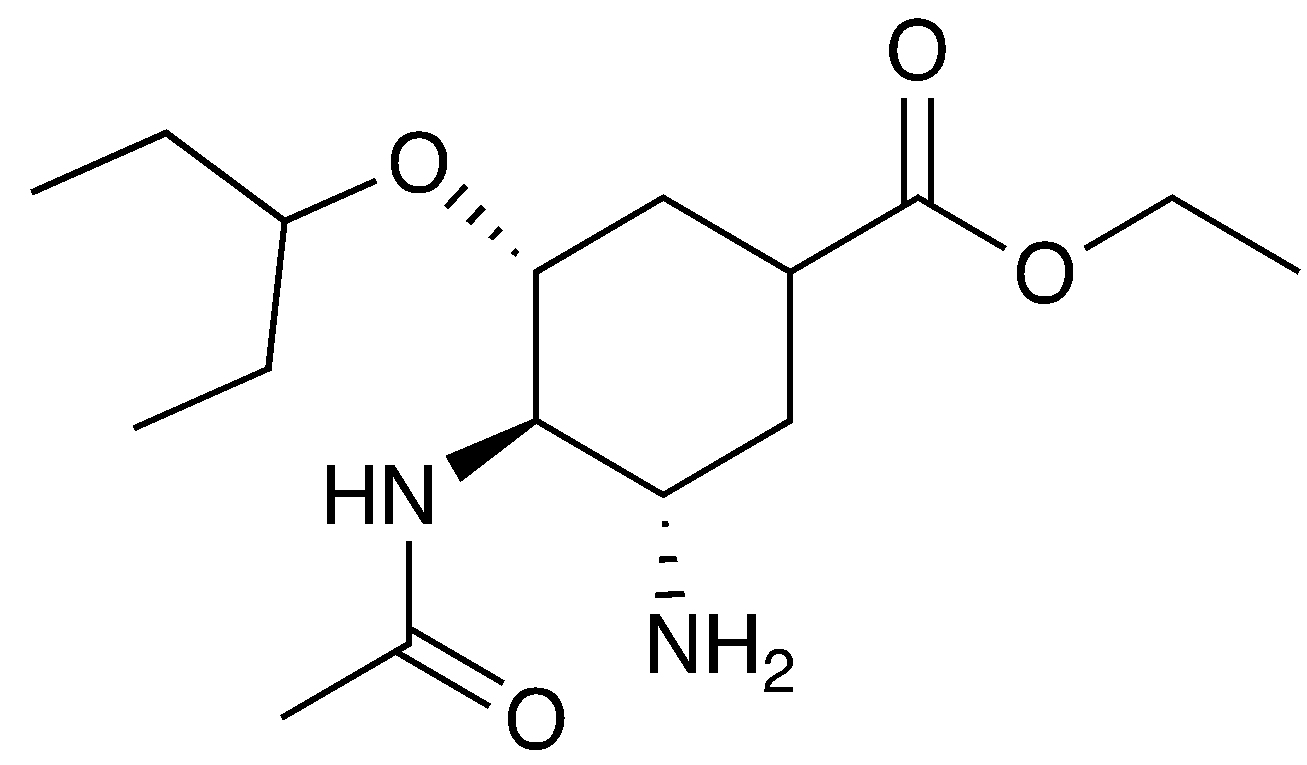
5. The drug oseltamivir (also known as Tamiflu; shown here) is an antiviral medication used to treat and prevent influenza. Which functional group(s) can you identify on this molecule? Circle and label the groups that you identify.
6. Rank the following carboxylic acids from least to most soluble in water. Provide a brief explanation for your ranking.
7. Methyldopa (also known as Aldomet) is a drug commonly used to treat high blood pressure, especially during pregnancy. Label the different forms of this drug as one of the following: carboxylate anion, carboxylate salt or carboxylic acid.
C. Saponification Reaction: The Reactants and Products Involved with the Synthesis of Soap
8. Use MolView to explore a 3D shape of a triacylglycerol, which is a fat or oil molecule, by entering “triacylglycerol” into the search window. Under the “Model” menu item you can explore different 3D views of this molecule (ball and stick, stick, etc.). Under “Jmol” menu item you can explore the charges on the molecule by clicking on “MEP surface lucent”. This electrostatic potential diagram uses red to indicate negative charge, blue to indicate positive charge and green for neutral or no charge.
One you’ve done that, identify the parts of the triacylglycerol molecules shown below including the glycerol backbone, the ester linkage and the hydrocarbon chains. Circle each of these three parts of a triacylglycerol and label your selections. Next, identify the three bonds that are cleaved in a saponification reaction to produce soap molecules.
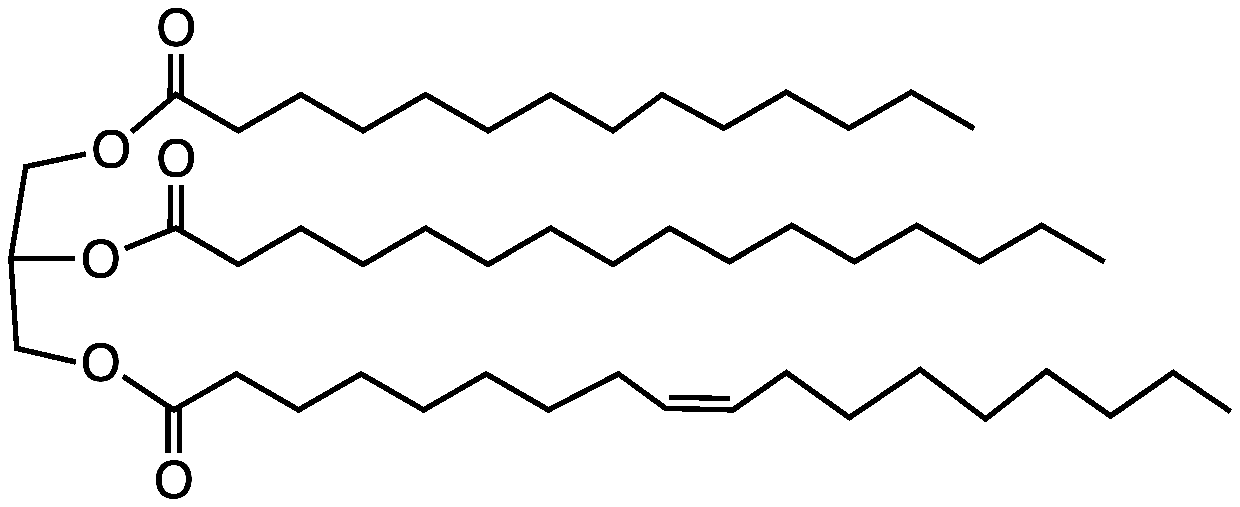
9. Soap is made by a saponification reaction-- the hydrolysis of an ester forms a carboxylate salt. Draw the products of the saponification reaction shown here:
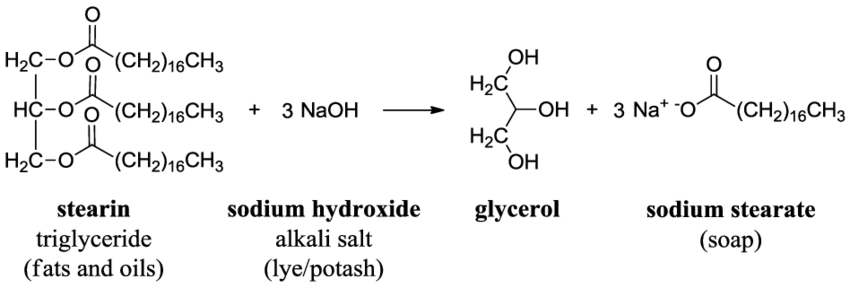
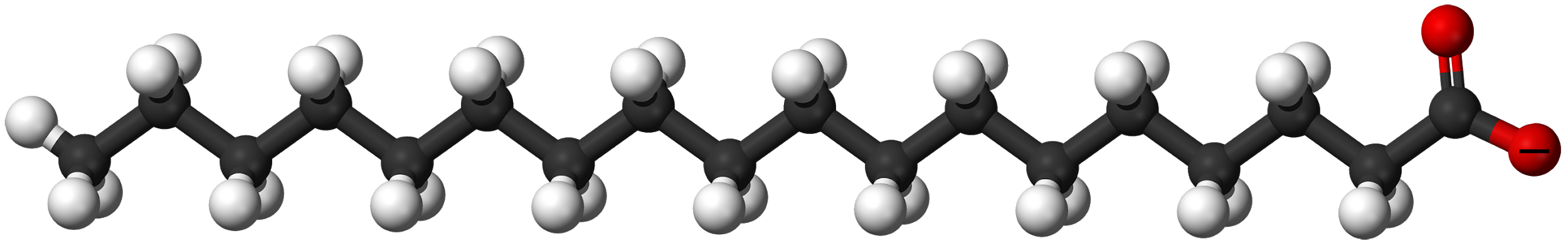
10. Use MolView to explore a 3D shape of the carboxylate salt that is produced in the reaction in question 9 called sodium stearate. This is a soap molecule! Under the “Model” menu item you can explore different 3D views of this molecule (ball and stick, stick, etc.). Under “Jmol” menu item you can explore the charges on the molecule by clicking on “MEP surface lucent”. This electrostatic potential diagram uses red to indicate negative charge, blue to indicate positive charge, and green for neutral or no charge. After exploring the 3D model complete the following:
On the sodium stearate compound below, draw water molecules where they would interact with this compound and label the partial charges and forces involved. Draw a minimum of five water molecules but even more would be better! Next, illustrate where the molecule butane (C4H10) would interact with sodium stearate. Draw a minimum of two butane molecules on your drawing and name the forces involved. Use either expanded structures for butane or line-bond drawings of butane.
D. How a Soap Functions – Micelle
11. Below is a representation of a micelle--the structure that results when soap is mixed with water. On the image of the micelle, draw water molecules to show how water is positioned in this situation. After that, add the word “oil” where you think oil would be in relation to the micelle.




