Be + F => BeF2 Name the ionic compound - ___________________________
Name Date Period
Type 1 (Ionic) Bonding Worksheet
For each pair of elements below draw electron dot structures showing the valence electrons in each atom. Then draw arrows to show where the valence electrons will go during a chemical reaction. Write the name of each type I ionic compound. Finally, fill in the n table below each reaction. Refer to the sample shown.
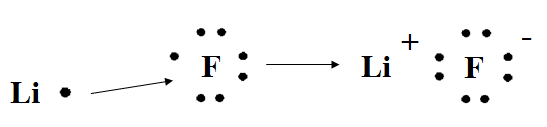
| Reactions- draw a picture showing each reaction & name the ionic compound | Atoms | Valence electrons | Electron transfer from/to each atom | Ions formed in the product |
Name the ionic compound – lithium fluoride | Li | Li loses 1 e- | Li 1+ | |
| F gains 1 e- | F 1- | |||
Name the ionic compound – calcium oxide | Ca | Ca loses 2 e- | Ca 2+ | |
| O gains 2 e- | O 2- | |||
| 3) Be + F => BeF2 Name the ionic compound - ___________________________ | ||||
Name the ionic compound - ___________________________ | ||||
Name the ionic compound - ___________________________ | ||||
| Reactions- draw a picture showing each reaction, write the chemical formula & name the ionic compound | Atoms | Valence electrons | Electron transfer from/to each atom | Ions formed in the product | |
Name the ionic compound - ___________________________ | |||||
Name the ionic compound - ___________________________ | |||||
Name the ionic compound - ___________________________ | |||||
Name the ionic compound - ___________________________ | |||||
Name the ionic compound - ___________________________ | |||||
| 10) Al + O => ____________________________ Name the ionic compound - ___________________________ | |||||
| 11) Li + O => ____________________________ Name the ionic compound - ___________________________ | |||||
| 12) K + S => ____________________________ Name the ionic compound - ___________________________ | |||||
| 13) Mg + O => ____________________________ Name the ionic compound - ___________________________ | |||||



