plss help i have to pass thistomorrow
Activity on: macromolecules, imf, liquids and solids
Answer the questions on another sheet of paper. Write your name on your paper and you don’t need to copy the questions. Submit your answers as pdf file.
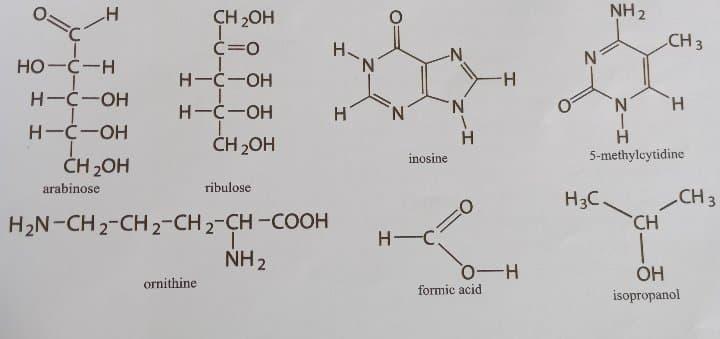
Macromolecules. Match the small molecules (see structures below) in column A, which serve as building blocks for macromolecules, with the type of compound in column B that best describes them. Write your answers as capital letters with the corresponding word answers.
Column A column B
Arabinose A. alcohol H. purine
Formic acid B. aldehyde I. pyrimidine
Inosine C. aldose
Isopropanol D. amino acid
5-methylcytidine E. carboxylic acid
Ornithine F. ketone
Ribulose G. ketose
![plss help i have to pass thistomorrow 1]()
Describe the intermolecular forces that must be overcome to convert these substances from liquid to gas: a) Cl2 b) NH3 c) BCl3
Which member in each pair has the greatest dispersion forces?
CH3OH or CH3CH2OH c) CH2Cl2 or CH2Br2
NH3 or N(CH3)3
Based on their composition and structure, list CH3COOH, CH3COOCH3, and CH3CH2OH in order of
Increasing intermolecular forces
Increasing viscosity
Increasing surface tension
Arrange the following substances in order of increasing volatility: CH4, CBr4, CH2CL2, CH3CL, CHBr3, CH2Br2.
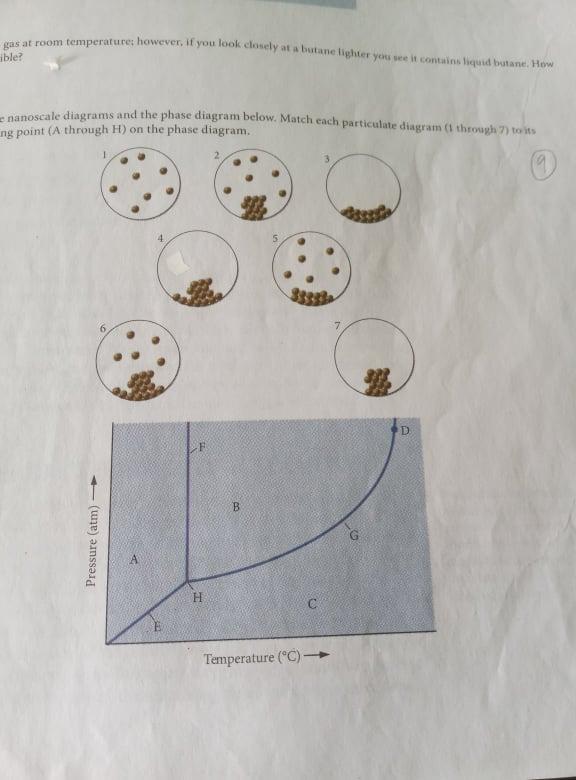
Examine the nanoscale diagrams and the phase diagrams below. Match each particulate diagram (1 through 7) to its corresponding point (A through H) on the phase diagram.
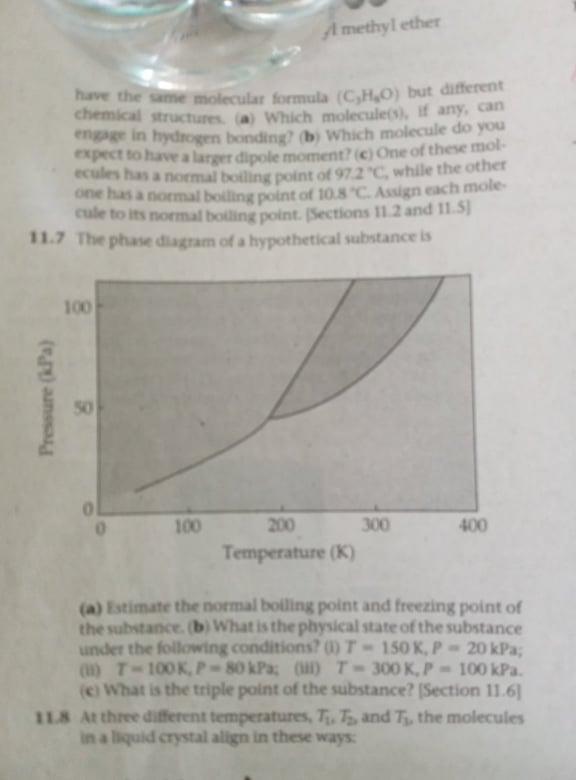
The phase diagram of a hypothetical substance is
![plss help i have to pass thistomorrow 3]()
Estimate the normal boiling point and freezing point of the substance.
What is he physical state of the substance under the following conditions?
T = 150 K, P = 20 kPa
T = 100 K, P = 80 kPa
T = 300 K, P = 100 kPa
What is the triple point of the substance?
Name the four different types of solids based on the types of bonds that holds the atoms together in place. Differentiate one from the other and give one example for each.
G o d B l e s s !