Lab question
Lab 3: Mixtures and Solutions
Pre‐lab Questions
Name two homogeneous mixtures and two heterogeneous mixtures that you come across daily.
Explain how you would separate a solid mixture of sand and salt using any of the methods mentioned above.
Experiment: Separation of a Mixture
A large amount of aspirin will dissolve in hot water, but very little (or none at all) will dissolve in cold water. The other ingredients in a safety‐coated aspirin pill are soluble in both hot and cold water. We can use this solubility difference to separate the aspirin in a pill from the other ingredients. You will allow a hot solution containing a crushed pill to cool in an ice bath, where the aspirin crystals should begin to form. If the cooled solution is filtered through a new piece of filter paper, almost all of the solid aspirin will be caught by the filter and the impurities that are soluble in cold water will remain in the water. 
Procedure
**Take photographs of your experiment set up for Part 1 and 2, and for your results. Submit them with your laboratory report.**
Prepare a boiling water bath.
HINT: This may be done over the stove.
CAUTION: Always use a heat pad or gloves when handling hot materials.
Pre‐weigh a sheet of filter paper and a watch glass. Record each mass in the Data section.
Prepare an ice water bath by filling a large bowl with a mixture of ice and tap water. Fill the wash bottle with distilled water and place it in the bath.
Mark three test tubes as A and B and C. Place Test tubes A and B into the test tube rack.
Prepare a filtering funnel as shown in Figure 3: fold a piece of filter paper in half twice to make quarters, and place the paper in the funnel so that three quarters are open on one side and one quarter is on the opposite side. Seat the filter paper into the funnel by moistening the paper with a small amount of water.
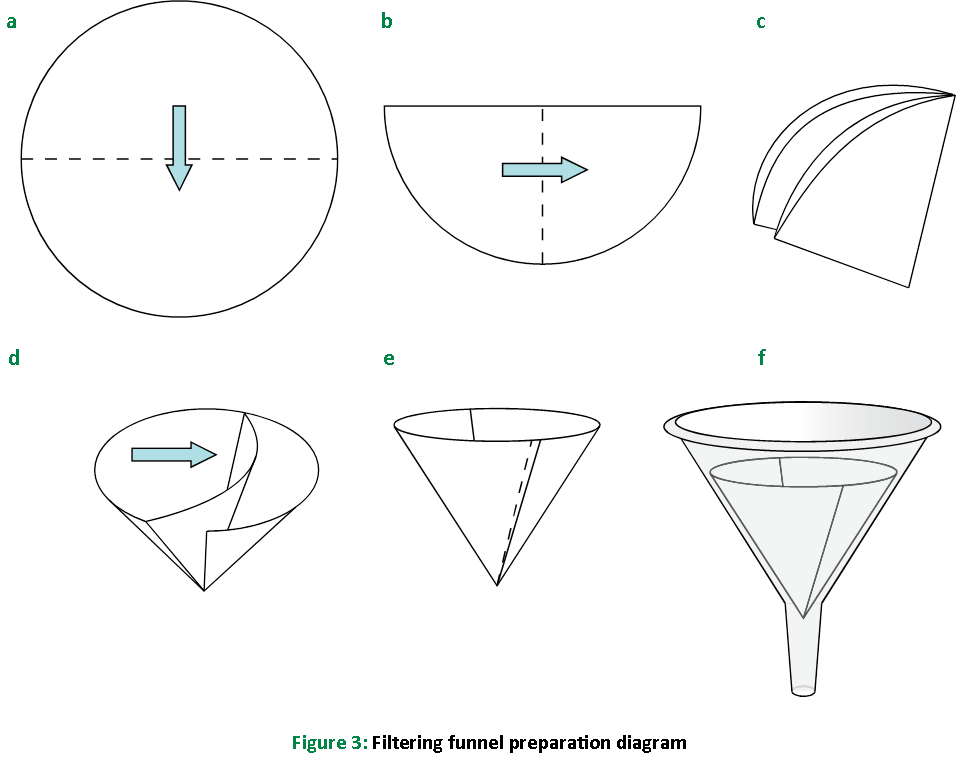
Insert the funnel with filter paper into test tube A supported in the rack. Discard any water that filters into this test tube.
Place four safety‐coated, low‐dose (81 mg) aspirin pills into the mortar. Gently crush the pills with the pestle into a fine powder.
Pour all of the ground aspirin powder into test tube C. HINT: Pour the powder onto a piece of paper, then shape the paper to more easily funnel the powder into the tube.
Add 10 mL water to the powder in the test tube and stir with a stirring rod. CAUTION: The test tube bottom is easily broken if too much force is applied with a stirring rod.
Use a test tube holder to place test tube C (with the aspirin powder) into the boiling water bath. Keep the test tube in the bath for 5 minutes. Stir the solution in the test tube frequently while it is heated. Record your observations in Table 1. Leave the test tube in the bath until the next step. CAUTION: This solution will be extremely hot. Always use a test tube holder to handle the heated test tube. Do not touch it directly with your hands.
Use a test tube holder to remove test tube C from the boiling water. Pour half the hot contents in the filtering funnel above test tube A, then immediately place test tube C back into the boiling water. Allow all the liquid to completely filter into the test tube.
Pour the rest of the hot contents into the funnel, and allow the solution to completely filter into test tube A. HINT: If filtering the first half of the solution went very slowly, it will help if you replace the filter paper for this new solution.
Allow the hot, filtered solution in test tube A to cool until it is no longer warm. Remove the filter paper and clean the funnel for later use.
Place test tube A with the cooled, filtered liquid into the ice water bath. Continue to cool the solution until generous amounts of crystals form. This should take about 5 minutes. If crystals do not form, try scratching the sides of the test tube with a glass stirring rod until the crystals begin to form. Record your observations in Table 1.
Prepare another folded filter paper (pre‐weighed) inside the cleaned funnel as you did in Step 5. Insert the cleaned filtering funnel into test tube B in the rack. Discard any water that filters into this test tube.
Transfer the crystals from cooled test tube A into the filtering funnel above test tube B. Use small amounts of ice‐cold distilled water from the wash bottle to rinse the crystals. DO NOT USE MORE THAN A SMALL AMOUNT OF ICE COLD WATER TO TRANSFER AND RINSE THE CRYSTALS OR TOO MANY WILL DISSOLVE.
Carefully remove the filter paper containing the crystals and place it on the watch glass, then set aside to dry. DO NOT THROW THE CRYSTALS AWAY. Allow the crystals to dry overnight or until the crystals are completely dry.
Clean up by washing any dirty glassware. Be sure that you do not throw away the crystals drying on the filter paper!
After the crystals are completely dry, weigh them on the scale (crystals plus filter paper and watch glass). Record the mass in the Data section, along with your observations in Table 1. DO NOT THROW THE CRYSTALS AWAY. THEY WILL BE USED FOR A LATER LAB.
To clean your mortar and pestle after the experiment, mix a teaspoon of baking soda with some warm water and gently scrub the surface with the test tube brush. Rinse thoroughly with water.
Data and Calculations
Pre‐weighed filter paper mass:
Pre‐weighed watch glass mass:
Filter paper, watch glass, and aspirin mass:
Mass of aspirin obtained:
Calculate the percent of recovery using the formula below:
![Lab question 3]()
Table 1: Observations for Aspirin Experiment
| Procedure Number | Observations |
| 10. Crushed aspirin and water mixture in hot water bath |
|
| 14. Aspirin mixture in ice water bath |
|
| 19. Aspirin crystals |
|
Post‐lab Questions
Take photographs of your experimental set up and results. Submit them with your laboratory report.
What important characteristics of aspirin did you use to separate it from the other pill components?
What is the purpose of rinsing the final aspirin crystals with a small amount of ice‐cold distilled water (Step 16)?
Evaluate your percentage recovery. Provide an explanation if your recovery was more than 100% or very low. How could you improve your results?
© eScience Labs, 2013



