Lab question and one discussion
Lab 6: Molecular Models
Pre-lab Questions
Identify the central atom and determine how many regions of electron density there are around the central atom in each of the following molecules:
Table 2: Pre-Lab Exercise
| Molecule | Central Atom | # Regions of Electron Density |
| BeCl2 |
|
|
| BH3 |
|
|
| CBr4 |
|
|
Explain why the shape of H2O is bent and not linear.
Experiment: Molecular Structures

Procedure
**Take photographs of your experimental set up for Part 1 and 2, and your results. Submit them with your laboratory report.**
Part 1: Magnets
Bring together the same poles of two bar magnets.
![Lab question and one discussion 2]()
Observe and describe what happens in Table 3.
Explore the linear shape of molecules by bringing the same poles as close together as you can in a straight line. Record your observations in Table 3.
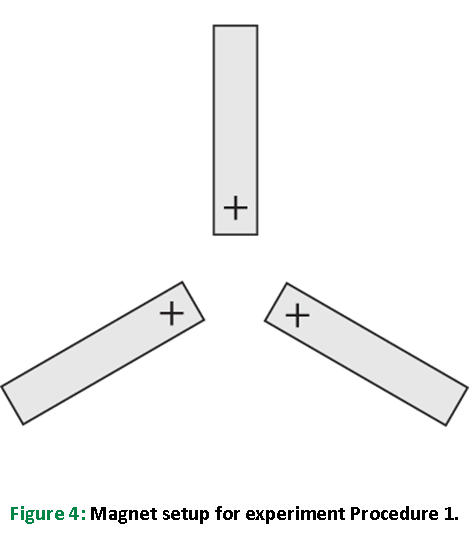
Explore why a molecule makes a trigonal planar shape by placing the same pole of 3 bar magnets as the points of an equilateral triangle with the rest of the magnet behind them.
Slowly bring the same pole of the 3 magnets as close as it is possible while maintaining the equilateral triangular appearance. Now try to find another shape that will be as stable with all of the magnets at least as close as they were in the equilateral triangle position. Record your observations in Table 3.
Part 2: Modeling Clay
Be sure to take a photograph of all of your models and submit them with your laboratory report.
Make a model of a molecule made up of two atoms such as H2 by attaching balls of modeling clay to both ends of a toothpick. Describe the shape in Table 4.
Make a model of a linear molecule that is made up of three atoms (such as CaCl2)using the following steps:
First attach two toothpicks to a larger ball of modeling clay representing the central atom. Locate them so that they are as far away from each other as possible.
Next attach two smaller balls of modeling clay to the ends of the two toothpicks.
Describe the shape in Table 4.
Determine the bond angle from one of the smaller balls of modeling clay to the large ball to another small ball using a protractor. Record the bond angle in Table 4.
Make models of the other molecular shapes listed in Table 4 (beginning with trigonal planar) by doing the following:
First attach a toothpick to a large ball of modeling clay (central atom) for each region of electron density from Table 1. Place them so that they are as far away from each other as possible.
Next attach a small ball of modeling clay for each bond in the molecule. You should create one ball for each bond listed in Table 1.
Remove the toothpicks that do not have small balls attached.
Describe and record the shape for all of the models in Table 4.
Use a protractor to determine the bond angle from one of the small balls to the large ball to another small ball. Record the bond angle in the Data section.
Table 4: Observations for Part 1
| Procedure Step | Observations |
| Step 2 | |
| Step 3 | |
| Step 5 |
Table 4: Observations and Data for Part 2
| Molecular Shape | Observations | Bond Angle |
| Step 2: Linear (2 atoms) |
|
|
| Step 3.c and Step 3.d: Linear (3 atoms) |
|
|
| Trigonal Planar |
|
|
| Bent |
|
|
| Trigonal Pyramidal |
|
|
| Tetrahedral |
|
|
| Trigonal Bipyramidal |
|
|
| Octahedral |
|
|
Post-lab Questions
Take photographs of your experiment set up for Part 1 and 2 (all models) and your results. Submit them with your laboratory report.
In Part 2 of the procedure, did your models fit the molecular shape description or match the geometry in the introduction? Explain why or why not.
Predict the shapes and bond angles of the following molecules:
BeCl2
BH3
CBr4
©eScience Labs, 2013