Lab question and one discussion
Lab 5: Electron Configuration
Pre-lab Questions
What is an electron configuration?
How is the light emitted by an atom related to its electron configuration?
Experiment: Chemistry of Fireworks
In this experiment the flame from a tea light candle is the outside energy source. The flame emits a broad range of energy, but the electrons of the atom being heated will only absorb specific amounts of energy.
In this experiment the flame from a tea light candle is the outside energy source. The flame emits a broad range of energy, but the electrons of the atom being heated will only absorb specific amounts of energy.
Procedure
**Take photographs of your experimental setup and results. Submit them with your laboratory report.**
Place a round piece of clay on the straight end of each inoculating loop. This will act as a holder.
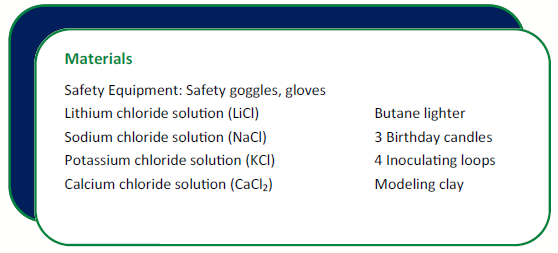
Place in order the LiCl, NaCl, KCl, and CaCl2 saturated solutions. Set one inoculating loop next to each sample.
Stabilize one birthday candle by placing the base of it in a piece of clay that is about 1 inch by 1 inch by 1 inch. The candle should stand freely and vertically (Figure 3). The other two candles are to be used if the first one burns down to the clay. CAUTION: Both lighter and candles can cause fire or burns to skin and/or clothing if the flame comes into contact with skin or clothes. Be sure you have your safety goggles on!
Use the butane lighter to light the stabilized birthday candle. Hold the inoculating loop for the LiCl at the very end of the non-looped end in order to avoid burns. Heat the looped end in the candle flame until its loop is faintly orange and any coating is burned off (Figure 3).
Dip the loop into the LiCl solution. CAUTION: The loop will remain extremely hot for several minutes following being in the flame. Do not touch the loop!
Bring the looped end of the inoculating loop into the flame. Make observations about what is happening, especially any color changes. HINT: The color change will be most apparent around the edges of the flame. You may have to try this a few times to see the color change.
Extinguish the candle and record your observations in Table 1.
Repeat the steps 4-7 for each of the other three solutions. Use a different inoculating loop for each one.
To clean-up, you may throw away the inoculating loops after they have cooled to room temperature.
*A demonstration video of the flame tests can viewed on the student portal.*
Data
Table 1: Results of Firework Material Ignition
| Substance | Observations |
| Lithium chloride |
|
| Sodium chloride |
|
| Potassium chloride |
|
| Calcium chloride |
|
Substance
Post‐lab Questions
Take photographs of your experimental setup and your results. Submit them with your laboratory report.
Write out the electron configurations of each of the metals of the salt compounds used and of carbon. Potassium is already done as an example for you. HINT: The periodic table is very helpful and can be used as guide.
Table 2: Results of Firework Material Ignition
| Element | Electron Configuration |
| K | 1s2 2s2 2p6 3s2 3p6 4s1 |
| Li |
|
| Na |
|
| Ca |
|
What is the approximate wavelength of light emitted by each of the salts?
| Salt | Color | Wavelength |
| LiCl |
|
|
| NaCl |
|
|
| KCl |
|
|
| CaCl2 |
|
|
Barium chloride emits a green color when flame tested. What can be said about the wavelength of light it emits?
©eScience Labs, 2013



