do the hw material engineering (( mechanical engineering hw))
1) Briefly (in a single phrase or short sentence) describe/define the following terms in your own words and, if relevant, include an example:
Polymorphous g) Microconstituent
Matrix h) Primary microconstituent
Dispersed phase i) Eutectic microconstituent
Dispersion strengthening j) Hypoeutectic alloy
Intermetallic compound k) Hypereutectic alloy
Intermediate solid solution l) Hot shortness
2) Use the hypothetical phase diagram at right to answer the following questions: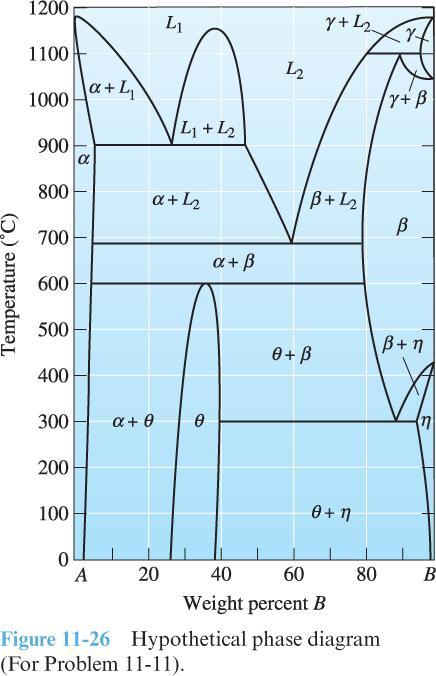
Identify all solid solutions.
Identify all intermetallic compounds, and determine if they are stoichiometric or non-stoichiometric.
Identify all three-phase reactions. For each, list the corresponding temperature, the reaction in equation form (i.e., X → Y + Z), the approximate composition of each phase in the reaction, and the type of reaction.
Is either constituent A or constituent B allotropic? If yes, describe why.
Use the Gibbs phase rule to verify that a three-phase reaction has zero degrees of freedom, a two-phase region of the phase diagram has one degree of freedom (what is it?), and a single-phase region of the phase diagram has two degrees of freedom (what are they?).
3) Use the phase diagram below for an aluminum-silicon (Al-Si) alloy to answer the following questions:
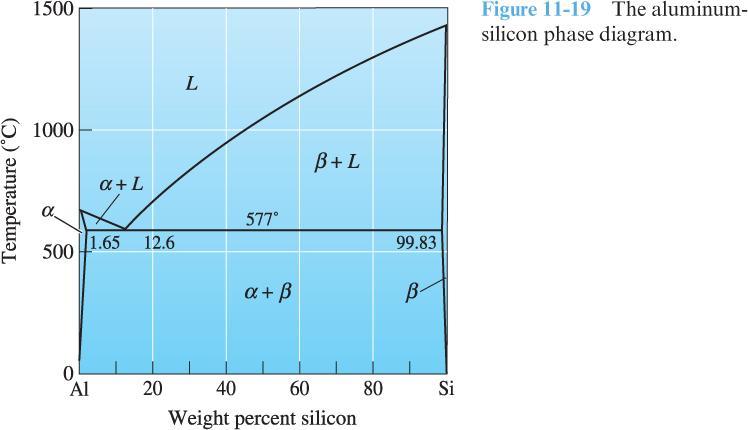
Is the phase diagram unary or binary? Why?
Is the phase diagram isomorphous or polymorphous? Why? Connect your answer to the solubility of the constituents (limited or unlimited), as predicted by the Hume-Rothery rules.
Are there any three-phase reactions? If yes, describe.
Label the solidus, liquidus, and solvus curves.
At what composition would you expect the ultimate tensile strength of the Al-Si alloy to be the greatest?
1.65% Si
12.6% Si
99.83% Si
The ultimate tensile strength does not vary with composition.
4) Use the phase diagram below for an aluminum-magnesium (Al-Mg) alloy to answer the following questions:
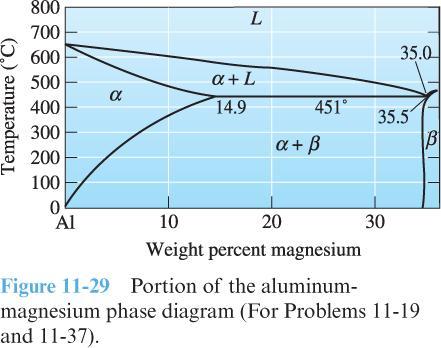
What is the solubility of magnesium in solid aluminum at 250 ◦C (in terms of wt. % Mg)? Describe what happens if this solubility limit is exceeded.
What is the maximum solubility of magnesium in solid aluminum (in terms of wt. % Mg)? At approximately what temperature does this occur?
5) Use the phase diagram below for a lead-tin (Pb-Sn) alloy to answer the following questions:
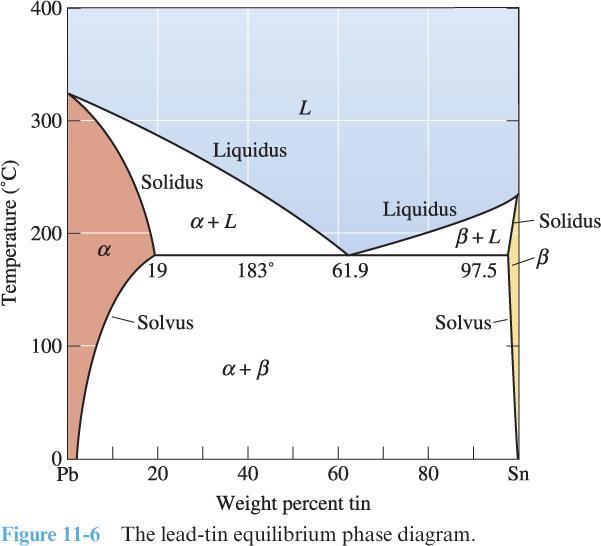
Consider molten lead-tin solutions at 375 ◦C with the following compositions:
Pb-1% Sn
Pb-10% Sn
Pb-40% Sn
For each case, describe (and perhaps illustrate) how the microstructure changes as the molten liquid is cooled to room temperature. (Use the words “nucleation” and “growth” in your discussion. Discuss the role of the liquidus, solidus, solvus, and/or eutectic, as applicable. What is the strengthening mechanism, i.e., solid-solution strengthening or dispersion strengthening?)
For the Pb-10% Sn alloy:
Estimate the composition of the first solid to form.
Estimate the composition and amount of each phase at 375 ◦C, 183 ◦C, and 20 ◦C.
For the Pb-40% Sn alloy:
Estimate the composition of the first solid to form.
Estimate the composition and amount of each phase at 375 ◦C, 184 ◦C, 182 ◦C, and 20 ◦C.
What composition has the lowest melting temperature?



