material science and engineering homework!
MEE 312 – Engineering Materials
Homework 9
Due Tuesday by April 25, 4:30 p.m. in my mailbox or MEE Office
1) Briefly (in a single phrase or short sentence) describe/define the following terms in your own words and, if relevant, include an example:
Age/precipitation hardening g) Pearlite
Solution treatment h) Bainite
Supersaturated solid solution i) Martensite
Austenite j) Austenitizing
Ferrite k) Annealing (steel)
Cementite l) Normalizing
2) Compare and contrast coherent and non-coherent precipitates.
3) Complete the table below, which summarizes the characteristics of different strengthening mechanisms in alloys accomplished via solidification and/or heat treating.
| Strengthening Mechanism | Number and Type of Solid Phases | Relation to Solubility of Alloy | Strengthening Effect |
| Solid-solution strengthening | Either unlimited solubility, or solubility limit not exceeded | Low | |
| Dispersion strengthening | |||
| Precipitation/age hardening | Two solid phases, one a coherent precipitate |
4) Discuss the three stages of age hardening, as illustrated in Fig. 12-9 of your textbook for an Al-4% Cu alloy. Describe the role of the (coherent) non-equilibrium precipitates GP-I, GP-II, and θ’ in this process. Also explain the role of time and temperature, as illustrated in Fig. 12-12.
5) Suppose that in Problem 4 the Al-4% Cu alloy is held at the aging temperature for a sufficiently long time so that the (non-coherent) equilibrium precipitate θ is produced. This is referred to as overaging. How does overaging affect the strength of the alloy? How does the resulting microstructure of the overaged alloy differ from an alloy that is slow-cooled to the aging temperature from its molten state? (Hint: Refer to the microstructures in Fig. 12-5.) Would you expect the slow-cooled alloy or overaged alloy to be stronger, assuming a small overaging time?
6) Compare and contrast artificial aging with natural aging.
7) First fill-in the two-phase regions in the hypothetical phase diagram at right. Then determine which of the following alloys might be good candidates for age hardening, and explain your reasoning. For those that are good candidates, describe the heat treatment required and include recommended temperatures.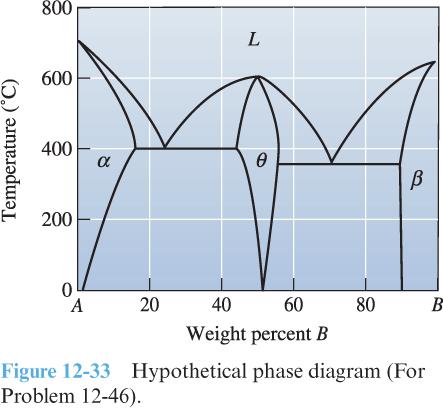
a) A-10% B
b) A-30% B
c) A-55% B
d) A-95% B
8) Use the phase diagram for an iron-carbon (Fe-C) alloy at right to answer the following questions: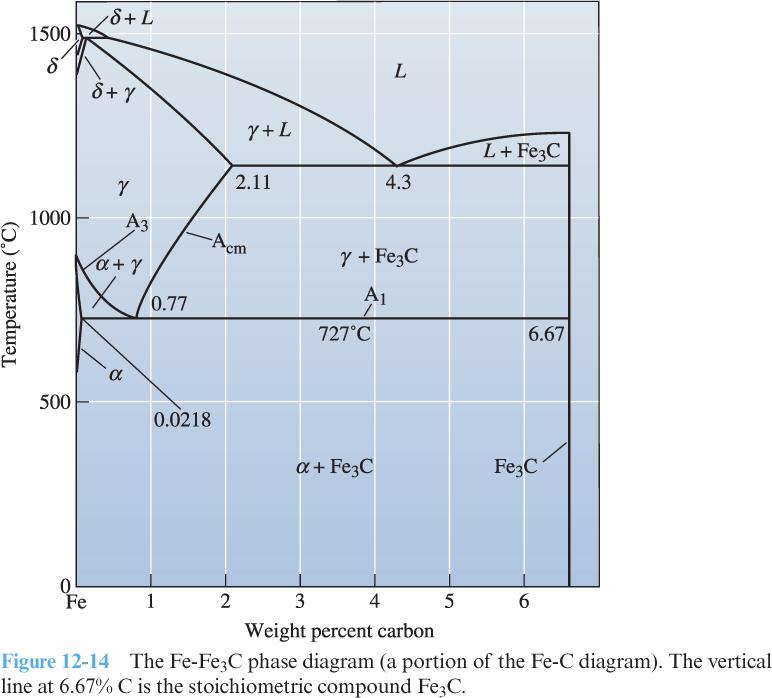
Identify all solid solutions.
Identify all intermetallic compounds, and determine if they are stoichiometric or non-stoichiometric.
Identify all three-phase reactions. For each, list the corresponding temperature, the reaction in equation form (i.e., X Y + Z), the approximate composition of each phase in the reaction, and the type of reaction.
Label the solidus, liquidus, solvus, eutectic, and eutectoid curves.
Define the A1, A3, and Acm temperatures.
Identify the eutectoid composition, the hypoeutectoid region, the hypereutectoid region, and composition (wt. % C) where steel transitions to cast iron.
Describe the microstructural evolution (in terms of phases and microconstituents – make sure you know the difference!) of a Fe-0.6% C hypoeutectoid steel as it cools from 900 ◦C to 400 ◦C. (Hint: Refer to Fig. 12-16 and the accompanying discussion for guidance.)
9) Complete the table below, which summarizes the effect of different heat treatments on the mechanical properties of steel. For the eutectoid microconstituents, your options are coarse pearlite (CP), fine pearlite (FP), bainite (B), and tempered martensite (TM). For the other questions, your options are low (L), moderate (M), high (H), and very high (VH).
| Heat Treatment | Cooling Rate | Eutectoid Microconstituent | Strength |
| Austenitize and oven cool | |||
| Austenitize and air cool | |||
| Austenitize and quench in hot oil | |||
| Austenitize, quench in cool water, then temper |
Compare and contrast the structure of the three key eutectoid microconstituents (pearlite, bainite, and tempered martensite); refer to Fig. 13-2 in your textbook, and discuss how the soft ferrite and hard cementite phases are arranged in each type of microconstituent. (Again, make sure you recognize the difference between phase and microconstituent.)



