Chemistry Homework
Lab 9: Chemical Reactions II
Pre-lab Questions
What is a limiting reagent?
A student used 7.15 g of CaCl2 and 9.25 g of K2CO3 to make CaCO3. The actual yield was 6.15 g of CaCO3. Calculate the limiting reagent and the percent yield.
Experiment: Synthesis of Garden Lime

Procedure
**Take photographs of your experiment set up and your results. Submit them with your laboratory report.**
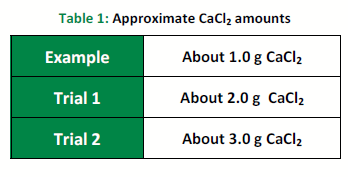
Table 1 provides an example set of data for 1.0 g CaCl2.
For Trial 1, weigh into a 250 mL beaker the amount of calcium chloride (CaCl2) shown in Table 1. Record the exact mass you weigh out in the Trial 1 column of the Data section.
Measure 50.0 mL of distilled water into a 100 mL graduated cylinder. Pour the water into the 250 mL beaker with the calcium chloride.
Stir the solution with a stirring rod until all of the calcium chloride is dissolved.
Weigh out 2.5 g of potassium carbonate (K2CO3) in a 50 mL beaker. Record the exact mass in the Data section.
Measure 25.0 mL of distilled water into a 100 mL graduated cylinder. Add the water into the 50 mL beaker containing the potassium carbonate.
Stir the potassium carbonate in the distilled water with a stirring rod until it is all dissolved.
Pour the K2CO3 solution into the 250 mL beaker that has the CaCl2 solution. Rinse the beaker that contained the K2CO3 with a few mL of water and add this to the CaCl2 solution. Stir the mixture.
As soon as the reaction begins, record your observations in the Data section. Continue stirring until you see no more precipitate forming.
Set up the funnel in the Erlenmeyer flask as shown in Figure 2.
HINT: Do NOT begin filtering yet!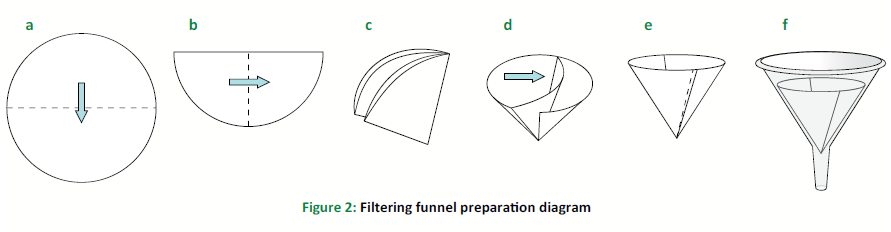
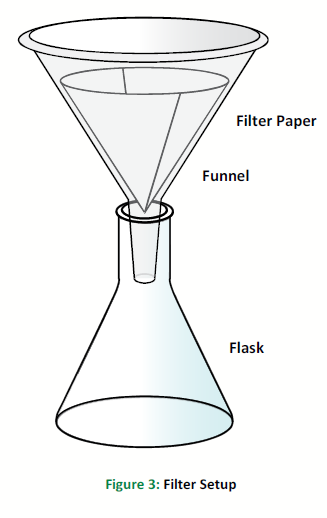
Zero the scale and weigh a piece of filter paper and a watch glass. Record the masses of both items in the Data section.
Prepare a filtering funnel as shown in Figure 2: fold a piece of filter paper in half twice to make quarters, and open the paper to make a small cone (three quarters are open on one side and one quarter is on the opposite side). Place the paper cone into the funnel and hold it in place with your fingers. Pour a small amount of distilled water through the paper to secure it inside the funnel.
Filter the mixture by pouring it into the filter paper in the funnel. Use the stirring rod and distilled water in a wash bottle to transfer the entire solid into the filter paper.
HINT: For best results, be sure to transfer all of the precipitate into the filter paper. Use a rubber policeman if it is available to help with the transfer.
Rinse the remaining solid in the filter paper twice with distilled water from a wash bottle to rinse off excess sodium chloride (NaCl). After all the liquid has filtered through, rinse the product with approximately 5 mL of ethanol to aid in its drying. Allow the ethanol to completely finish filtering through the paper.
Remove the filter paper carefully so as to not lose any product. Gently unfold the filter paper and lay it flat on the pre-weighed watch glass to dry.
Allow the product to air dry completely. This may take 24 hours or more. Once dry, weigh the dry product on the filter paper and watch glass. Record the total mass in the Data section. Calculate the mass of the product.
Repeat the above procedure for Trial 2 using the amount of CaCl2 mass indicated in Table 1.
To clean up, wash any dirty glassware, pour liquids down the drain, and throw the product on the filter paper in the trash.
Data
Record your data for each of the trials in Table 2.
Record your reaction observations (Step 8) below:
Table 2: Reaction Product Data
| Mass (g) | Example | Trial 1 | Trial 2 |
| Mass of CaCl2 | 1.0 g | ||
| Mass of K2CO3 | 2.5 g | ||
| Mass of filter paper | 0.8 g | ||
| Mass of watch glass | 38.5 g | ||
| Combined mass of product, filter paper, and watch glass | 40.2 g | ||
| Mass of dry product | 0.9 g |
Calculations
Determine the limiting reagent for each trial. Show your calculations.
Hint: See the example in the Introduction.
Example:
Trial 1:
Note: These should be about the same and either CaCl2 or K2CO3 can be the limiting reagent depending on their initial masses.
Trial 2:
Table 4: Comparison of Theoretical and Actual Yields for CaCO3
| Trial # | Limiting Reagent | Theoretical Yield of CaCO3 | Actual Yield of CaCO3 | % Yield |
| Trial 1 | | | | |
| Trial 2 | | | | |
| Trial 3 | | | | |
Calculate the theoretical yield of CaCO3 that could be produced by each trial and then fill in Table 2.
Find the percent yield each trial obtained for the CaCO3.
Post-lab Questions
Take photographs of your experiment set up and you results. Submit them with your laboratory report.
Compare the results of the different trials. How does the amount of grams of CaCO3 compare?
Were the results of the trials as you expected? Why or why not?
Predict what would happen if 6.0 grams of CaCl2 were used for the reaction and the amount of K2CO3 remained the same.
©eScience Labs, 2013



