lab report chemistry
Synthesis of E,E-dibenzalacetone (E,E-DBA)
Afra ALMashari
5/18/2017
Abstract
The preparation of E,E-dibenzalacetone (Scheme 1), from benzaldehyde and acetone in the presence of ethanol and Ethanolic sodium hydroxide, is described. E,E-dibenzalacetone was purified by recrystallization, E,E-dibenzalacetone was recrystallized from. E,E-dibenzalacetone was obtained in 67.1% yield. The crude and purified products were characterized by melting point determination.
Introduction
The purpose of this experiment was to employ a based-catalyzed aldol condensation in the synthesize of E,E-dibenzalacetone and to characterize the crude and purified samples of E,E-dibenzalacetone by melting point determination. As shown in Scheme 1 this involved allowing benzaldehyde to react with acetone in the presence of ethanol and Ethanolic sodium hydroxide.
Scheme 1 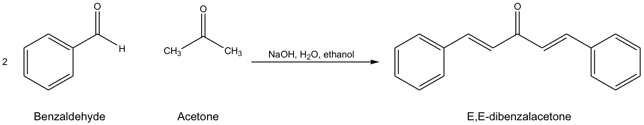
Results
The results of the synthesis of E,E-dibenzalacetone are summarized in Table 1.
| Compound | molar mass (g/mol) | volume/mass moles | yield | Melting point (C) |
| benzaldehyde (density 1.043 g/mL) | 106.2 g/mole | 0.25 ml 0.26 g 0.002 mole | N/A | N/A |
| acetone (density 0.788 g/mL) | 580.08 g/mole | 0.1 ml 0.07 g 0.001 mole | N/A | N/A |
| E,E-DBA (a yellow solid) | 234.24 g/mole | N/A | Theoretical 0.234 g 0.001 mole | Literature value 110-111oC |
| E,E-DBA (a yellow solid) | 234.24 g/mole | N/A | Actual crude* 0.645 g 63.7% purified** 0.14 g 67.1 | Actual Crude 109-110 Oc purified 110-111 Oc |
* a yellow solid ** a yellow crystals solid
Discussion
Experimental Procedure



