Attached file
Unit 3: Chemical Reactions: Conservation of Matter and Energy
Test Scoring Guide
Mrs. Marais
Each standard will be graded on a 4-3-2-1 basis with:
“4” indicating you are “excelling for this standard” and represents an A (90% or above).
“3” indicating you are “meeting standard” and represents a B (80%).
“2” indicating you are “approaching standard” and represents a C (70%).
“1” indicating you are “beginning for this standard” and represents an F (below 70%).
This test will be divided into two parts.
PS 7: Consists of 11 free-response questions.
PS 4: Consists of 10 free-response questions.
You can see each standard(s) below and accompanying rubrics to assess your understanding of these standard(s).
You will receive a 4-3-2-1 grade for each standard(s) using the rubric below.
Your will receive a weighted score for each standard.
For this test:
PS 7: weighted 50 points
4 – 90% (45/50)
3 – 80% (40/50)
2 – 70% (35/50)
1 - < 70% (less than 35 points)
PS 4: weighted 50 points
4 – 90% (45/50)
3 – 80% (40/50)
2 – 70% (35/50)
1 - < 70% (less than 35 points)
The weighted score for each standard(s) will be added together for a total score.
You must meet standard or achieve a “3” on each standard(s).
Please email me directly if you do not achieve a “3” on each standard(s) and we will make a plan to help you meet this requirement.
You have two options to complete this standards-based assessment.
Option 1: OPEN, SAVE, COMPLETE in Microsoft Word (using Microsoft Paint to create images/diagrams as needed), and then UPLOAD.
Option 2: OPEN, PRINT, COMPLETE by-hand, SCAN/TAKE a PIC, and then UPLOAD. NOTE: If you choose to do this assignment by hand, please print VERY neatly and draw figures carefully! If I can’t read/understand your work, I will need to give your response a “zero.”
Priority Standard:
PS 7
HS-PS1-7: Use mathematical representations to support the claim that atoms, and therefore mass, are conserved during a chemical reaction.
| 4 | 3 | 2 | 1 | |
| Excelling Excelling means the student has thoroughly demonstrated knowledge on standard assessed. | Meeting Meeting means the student has demonstrated competence on standard assessed. | Approaching Approaching means the student has partially demonstrated competence on standard assessed. | Beginning Beginning means the student has not yet demonstrated competence on standard assessed. | |
| Correctly and clearly answers questions 1-7. Shows deep understanding. AND Correctly and clearly answers questions 8 – 10. AND Correctly and clearly answers OR makes a reasonable attempt to answer question 11. You do NOT need to get question 11 right to obtain a “4” but you do need to make a reasonable attempt. | Does not meet requirements for level 4. Correctly and clearly answers questions 1-7. | Does not meet requirements for level 3.
Shows some understanding from responses of conservation of particles and/or mass. | Does not meet requirements for level 2. OR Understanding cannot be determined as answers are vague, incomplete, missing, or incomprehensible. |
PART 1: Questions 1-11 below will be evaluated using the rubric above for priority standard 7.
Consider the reaction below:
Mg(s) + HCl (aq) MgCl2 (aq) + H2 (g)
IDENTIFY the reactants for this reaction and their states.
Magnesium (solid) , Hydrogen (gas) and chlorine (gas)
IDENTIFY the products for this reaction and their states.
Hydrogen is gas
DRAW, LABEL, and COLOR-CODE representative particles for each reactant(s) and each product(s).
(Complete this section to make a color-code key)
Atom Mg is denoted by the color:
Atom H is denoted by the color:
Atom Cl is denoted by the color:
| Reactant(s) | Product(s) |
A chemistry student carries out the reaction between Mg metal and HCl acid using the set-up below.
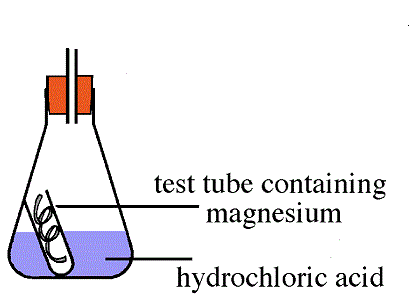
The student first places the empty flask, empty test tube, and stopper with tube on an electronic balance and “zeroes” the balance.
The student adds hydrochloric acid to the flask, magnesium to the test tube, replaces the stopper with tube, and places on the electronic balance again and records a mass of 9.63 grams.
The student then tips the flask so that the magnesium metal moves from the test tube into the flask and places the set-up back on the electronic balance again.
The student waits for the reaction to run to finish (e.g. stop producing hydrogen gas).
The student records the final mass on the electronic balance as 9.43 grams.
Answer these questions:
EXPLAIN why the mass was different BEFORE and AFTER mixing magnesium with hydrochloric acid.
Before it was empty, there was a zero balance after adding magnesium and hydrochloric acid
DETERMINE what mass of hydrogen gas was produced in this reaction. SHOW ALL YOUR WORK CLEARLY.
DETERMINE how many moles of hydrogen gas were produced in this reaction. SHOW ALL YOUR WORK CLEARLY.
If the student wanted to produce the same amount of hydrogen gas in another experiment, DETERMINE how many moles of Mg they would need to use and DETERMINE what mass of Mg they would then need to weigh out. SHOW ALL YOUR WORK CLEARLY.
HINT: What is the mole-to-mole relationship between H2 and Mg in your balanced chemical equation?
NOTE: You are NOT required to get this question right to achieve a “4” but you are required to make an attempt.
NOTE: You are MOST welcome to email me for hints!!!
Priority Standard
PS 4
HS-PS1-4: Develop a model to illustrate that the release or absorption of energy from a chemical reaction system depends upon the changes in total bond energy.
| 4 | 3 | 2 | 1 | |
| Excelling Excelling means the student has thoroughly demonstrated knowledge on standard assessed. | Meeting Meeting means the student has demonstrated competence on standard assessed. | Approaching Approaching means the student has partially demonstrated competence on standard assessed. | Beginning Beginning means the student has not yet demonstrated competence on standard assessed. | |
| Correctly and clearly answers questions 1-4 and questions 6-9. AND Correctly and clearly answers questions 5 and 10. It is clear that student has mastered how to represent ENDO/EXOthermic reactions using energy diagrams. | Does not meet requirements for level 4. Correctly and clearly answers questions 1-4 and questions 6-9. Correctly and clearly answers questions 5 and 10 or answers with only minor mistakes. It is clear that student understands how to represent ENDO/EXOthermic reactions using energy diagrams. | Does not meet requirements for level 3.
Correctly and clearly answers 6/10 questions. OR Shows some understanding of EXOthermic/ENDOthermic reactions from responses. | Does not meet requirements for level 2. OR Understanding cannot be determined as answers are vague, incomplete, missing, or incomprehensible. |
PART 2: Questions 1-10 below will be evaluated using the rubric above for priority standard 4.
Study the generic reaction below.
AB + CD AD + CB
When this reaction is conducted in a plastic cup, the cup feels warmer after the reaction.
IDENTIFY whether this reaction is ENDOthermic or EXOthermic. PROVIDE a scientific explanation to support your decision.
COPY the generic reaction above and place the term “HEAT” on the correct side of the equation. PROVIDE a scientific explanation to support your decision.
PREDICT the sign of ΔH.
EXPLAIN “why” this reaction is ENDOthermic/EXOthermic. Your answer should compare the energy changes associated with breaking bonds in the reactants with energy changes associated with forming bonds in the products.
SKETCH a hypothetical energy diagram for this reaction (see blank energy diagram below).
LABEL reactants, products, transition state, activation energy (label Ea), and change in enthalpy (label ΔH).
NOTE: You do NOT need to include actual values.
PROVIDE a brief 2-3 sentence caption explaining how your energy diagram shows that this is an EXO/ENDOthermic reaction.
Study the generic reaction below.
AX + BY AY + BX
When this reaction is conducted in a plastic cup, the cup feels colder after the reaction.
IDENTIFY whether this reaction is ENDOthermic or EXOthermic. PROVIDE a scientific explanation to support your decision.
COPY the generic reaction above and place the term “HEAT” on the correct side of the equation. PROVIDE a scientific explanation to support your decision.
PREDICT the sign of ΔH.
EXPLAIN “why” this reaction is ENDOthermic/EXOthermic. Your answer should compare the energy changes associated with breaking bonds in the reactants with energy changes associated with forming bonds in the products.
SKETCH a hypothetical energy diagram for this reaction (see blank energy diagram below).
LABEL reactants, products, transition state, activation energy (label Ea), and change in enthalpy (label ΔH).
NOTE: You do NOT need to include actual values.
PROVIDE a brief 2-3 sentence caption explaining how your energy diagram shows that this is an EXO/ENDOthermic reaction.




