An introduction of a chemistry lab report
Nanoscience lab report summer 2016
Introduction
Carbohydrate are an important part in biological system, which can be found inside and on the surface of cells. Carbohydrates play important role with biological recognitions that have the ability to bind proteins and play important role on cell surface. understanding of Carbohydrate protein interactions helps to produce medicine which improve immunity response and overcome on bacteria or leads to find ways to cure chronic diseases such as cancer1.
In professor Stine lab, the study of carbohydrate protein interactions relies on the formation of self-assembled monolayers(SAMs). The formation of SAMs occur on dealloyed Gold surface to make strong bond with thiol substrates. Then, proteins interact with terminated functional group on alkanthiols. In this work, the formation of Gal PEG SAM modified np-Au monolith used to capture Soybean agglutinin(SBA) from its mixture with Con A and release it afterward.
Materials:
Used materials in this experiment are β Gal-C12-SH (12-mercaptododecyl B-D-galactopyranoside), PEG (Poly Ethylene glycol), SBA (Soybean agglutinin), D Galactose , Concanvalin A Fluorescein isothiocyanate (Con A) , HPLC Etoh , and Tris (hydroxyethyl) amino methane (Tris Nacl buffer Ph~7.4 ).
Methods:
Np-Au preparation:
the experiment begins with preparation of dealloyed Gold after cutting gold plates(41.7% Au, 20.3% Ag and 38.0 % Cu) into 4 mm x 4 mm x 0.5 mm. Then, dealloyed Au plates by using nitric acid for 48 hours and after the first 24 hours, it has to be refilled again with nitric acid. After 48 hours, gold plates rinsed with Milli-Q water to neutralize their PH.
Preparation of Gal PEG SAMs and circulating SBA and Con A protein.
Second step, two of np-Au held in flow tube with spacers to close tightly and allow the flow go through Np-Au as well. Then, washing Gold plates and dry it by N2 gas then wash it with Etoh for 10 Mins. next step is the formation of Gal PEG SAM modified np-Au monolith by circulating Gal SAM mixture of 2 Microliter of β Gal-C12-SH and PEG (0.10: 0.90 mole fractions respectively) for 6-24 hrs. Then, setting UV detector at 280 nm and start washing it with Tris-Nacl buffer following by circulating mixture of 2 ml mixture of ConA-FITC and SBA (10 Mm of each in Tris Nacl buffer). Final step, release of SBA by 2 ml of 0.10 M of D Galactose in Tris Nacl buffer and detect capturing of SBA by UV detector at 280 nm .
result:
Protein interact with viruses, bacteria, fungi, and yeast decorated with sugars3. Therefore, comprehensive study of sugars is effective way of characterizing new lectins due to the importance of lectins in glycobiology and glycomics4. The latest development of the use of separation column is the use of monolithic material. It is useful way to study purification of lectin and how protein bind to sugar by modifying porous polymer monoliths with gold nanoparticles to form SAMs by building alkanethiols which have terminated functional group to bind proteins4. NP-Au monolith designed facilitate the formation of SAMs and the enter and exit of lectins for separation process. Moreover, using UV detector can provide an evidence of The detection of lectin molecules into Np-Au monoliths by using flow though method. After building GAL PEG SAM due to the strong formation of gold and alkane thiol bond. Next step is the circulation of SBA and Con A. SBA and Con A has the same molecular weight of 120 kDa4. SBA has higher conformational stability with monosaccharide than Con A , therefore Con A will show separation from SBA mixture and SBA will bind to Galacotose in the SAM. In figure 1, UV-vis scan of Con A and SBA mixture shows decreasing at 280 nm and 495 nm, Con A has two wavelengths at 280 and 4954 and SBA has only one wavelength at 280 (figure 2). As a result, the experiment set up with 280 nm to detect SBA releasing from the SAM. In figure 1, UV-vis scan shows SBA and Con A mixture before circulation and how it decreased after circulation due to the loading of SBA on Gal PEG SAM. In Figure 4 shows the decreeing of UV detector as a proof of loading SBA on SAM modified monolith at 280nm as well. Then, after the circulation of D-Galactose shows the increasing of UV reading due to the elution of SBA being captured by D Galactose and separated it from Gal PEG modified np-Au monolith in a period of around ten minutes.
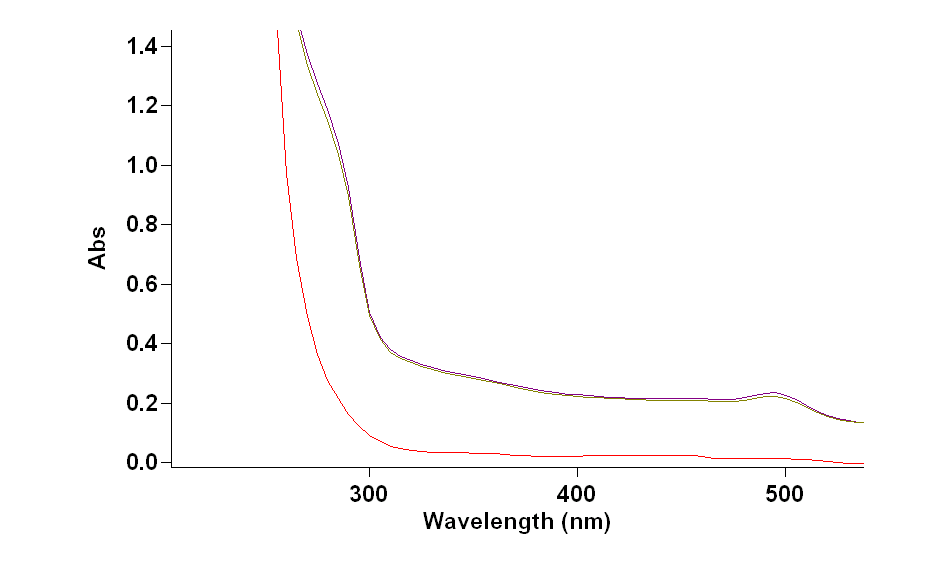
Figure 1: Con A and SBA before and after circulation by using UV-vis spectrum.
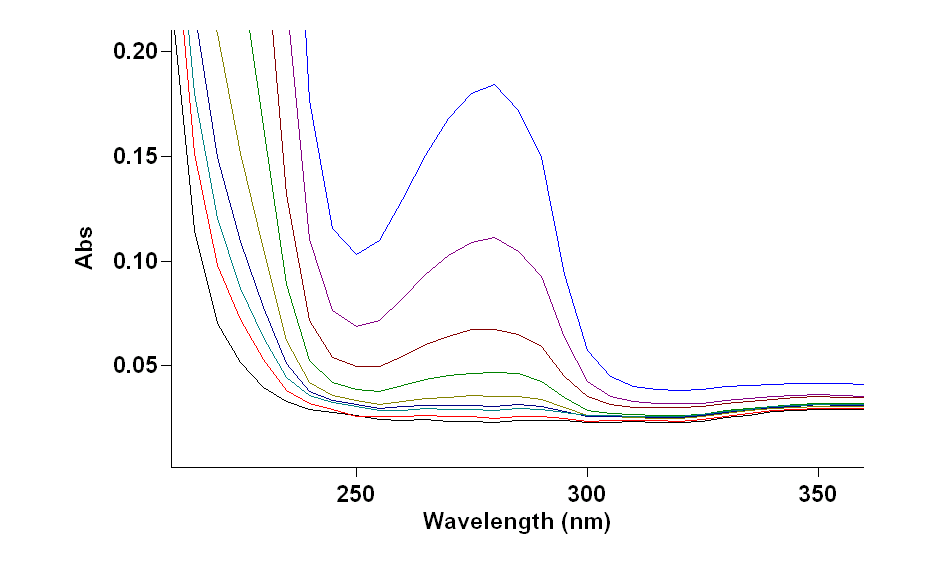
Fig 2: uv-vis scan of different concentration of SBA .
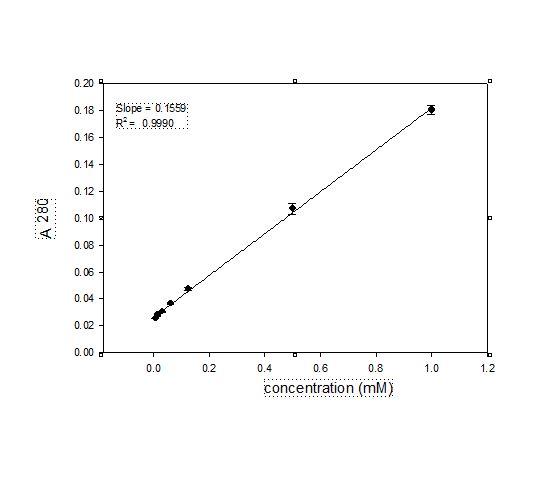
Fig 3:determination of extinction of coefficient of SBA at 280
Fig4: UV detector reading of the loading and releasing of SBA on Gal PEG modified np-Au monolith.
refrences
Debrassi, A.; de Vos, W.M.; Zuilhof,H.;Wennekes,T., Carbohdrate presenting Self-Assembled Monolayers. In Carbohydrate Nanotechnology, John Wiley & Sons, Inc: 2015; pp 1-51.
A.J.Alla, F.B.D’Andrea, J.K.Bhattari, J.A.Cooper, Y.H.Tan, A.V.Demchenko,K K.J. Stine, Selectivecapture of glycoproteins using lectin-modified nanoporousgoldmonolith, (2015) 19-30.
M.W Turner, The role of mannose-binding lectin in health and disease, (2003)
Allan.A.J.2016 unpublished thesis.



